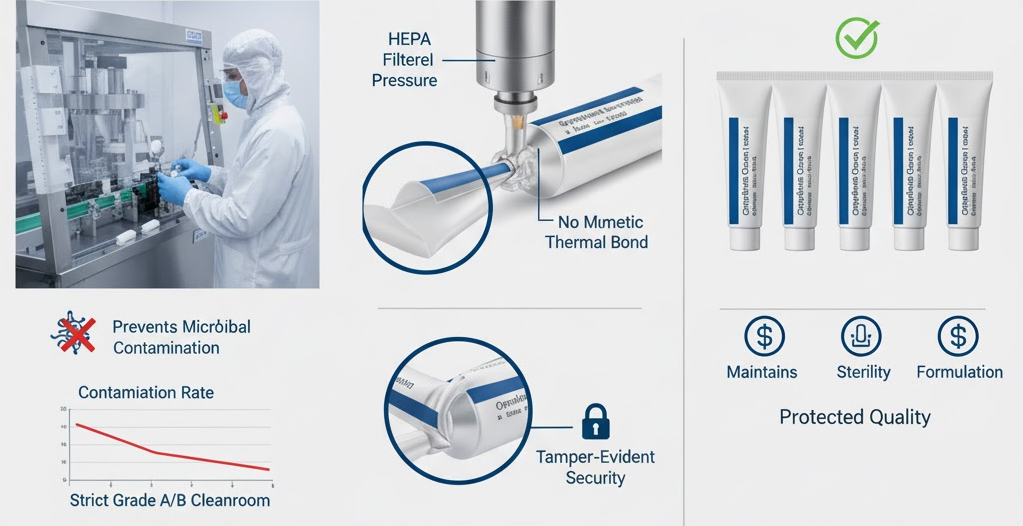
Ophthalmic ointments demand absolute precision in every production step. A single flaw in sealing can lead to contamination, leakage, or reduced drug potency — all of which directly affect patient safety. The seal is more than a packaging finish; it’s the final barrier that protects a sterile product from external exposure.
In this article, we’ll look at why proper tube sealing is essential in ophthalmic ointment manufacturing. You’ll learn how the right sealing technology preserves sterility, maintains drug stability, and helps meet strict regulatory standards.
Risk Factors in Ophthalmic Drug Packaging
Contamination Risk to the Eye and Product Safety
The human eye has very limited defenses against infection, which makes any microbial contamination in ophthalmic ointments especially dangerous. Studies back up this concern. In a prospective review of 410 multi-use eye medications, bacterial contamination was found in 5.6% of containers.
The issue was worse in ointments, which showed a 9.1% contamination rate, while eye drops were at 3%. These kinds of infections can lead to serious ocular conditions like keratitis, and in worst-case scenarios, endophthalmitis — especially risky given how sensitive the eye is to microorganisms.
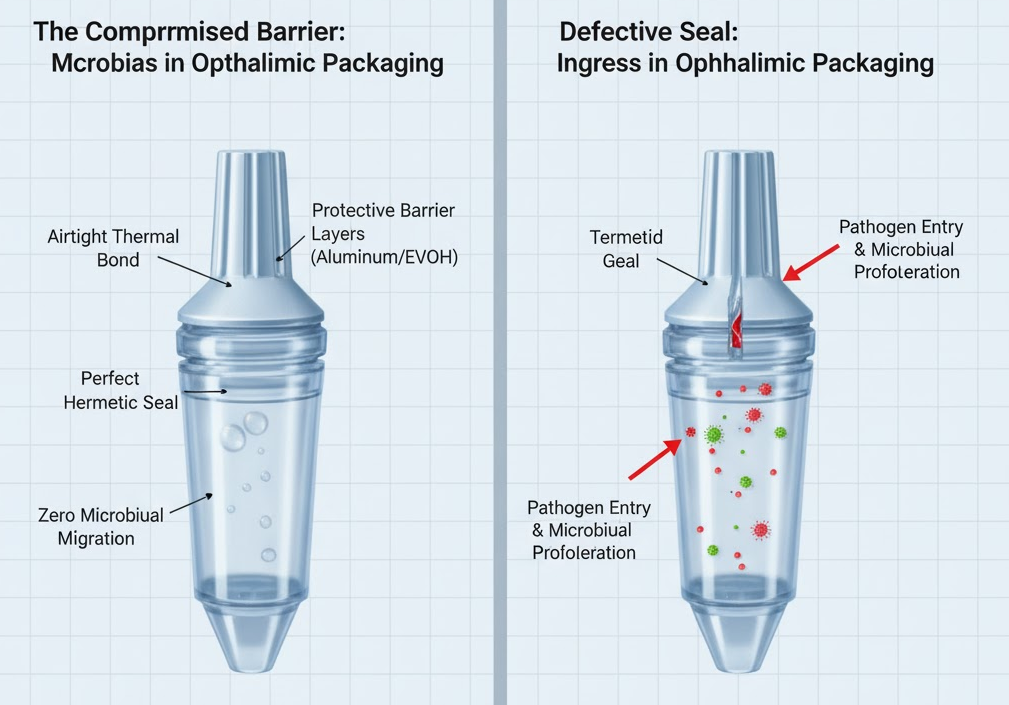
A failed or compromised tube seal is a major entry point for pathogens. When sealing is imperfect, even microscopic gaps allow bacteria or fungi to enter and proliferate. In semi-solid formulations like ophthalmic ointments, the viscous matrix can trap any contaminating organisms. Over time, moisture ingress through a faulty seal fosters microbial growth, which not only threatens patient safety, but can also degrade the drug formulation itself.
Leakage, Exposure, and Shelf-Life Failure
Seal failures aren’t limited to contamination — they also cause physical and chemical stability problems. Incomplete or poor-quality sealing can allow moisture vapor to pass through, upsetting the delicate balance of water content in an ointment. This change can lead to altered viscosity, phase separation, and loss of uniformity in the active pharmaceutical ingredients.
Exposure to air is another major issue. Many ophthalmic actives are sensitive to oxidation, and with a compromised tube, oxygen can slowly diffuse in. This degrades the active ingredients, reducing their potency and effectiveness. Over time, users may receive a lower-than-expected dose, which not only reduces therapeutic benefit but could also risk safety — especially for delicate eye conditions.
Storage and transport stress also amplify these risks. Temperature fluctuations, vibration, and mechanical pressure during shipping can further weaken inadequate seals. As the seal degrades, the rate of API breakdown or separation accelerates, shortening shelf life and potentially increasing the risk of product recalls or regulatory noncompliance.
Recommended Reading: Top 10 Syringe Filling Machine Manufacturers in 2025 – King Pack Machinery
Regulatory and Compliance Obligations (GMP / FDA / EU Guidelines)
Regulators treat ophthalmic products — especially sterile ointments — as high-risk. According to FDA guidance, manufacturers should include container closure integrity (CCI) testing in their stability protocols to ensure the packaging reliably maintains sterility over the product’s shelf life. The FDA considers a validated integrity test a more reliable indicator of long-term sterility than relying solely on periodic sterility testing.
In Europe, EU GMP Annex 1 explicitly requires that sealing operations for aseptic products be validated, and that container-closure systems undergo integrity testing. For containers closed by fusion (such as tube ends sealed by hot air or ultrasound), the regulation even recommends 100% integrity testing under certain conditions.
Because regulations demand rigorous testing, pharmaceutical manufacturers must maintain ongoing monitoring of their sealing lines. This includes validated container closure integrity testing, documented sampling plans, and continuous risk-based process control. Without this, firms risk noncompliance — which can lead to batch rejections, recalls, or worse, compromised patient safety.
Functional Role of Tube Sealing in Ointment Production
Ensuring Sterility and Barrier Protection
In ophthalmic manufacturing, sterility is not optional — it is the foundation of patient safety. Once the ointment is sterilized, either through aseptic filling or terminal sterilization, the packaging must preserve that sterile state until the product reaches the user. The final seal becomes the last defense against microbial entry and environmental exposure.
KPGFW-160 Filling and sealing machine – King Pack Machinery
Any compromise in this stage risks contamination that can cause severe ocular infections or regulatory noncompliance. Modern filling lines are designed to support this critical barrier function. Hot air sealing and ultrasonic welding create consistent, hermetic joints that prevent ingress of air, moisture, or particulates.
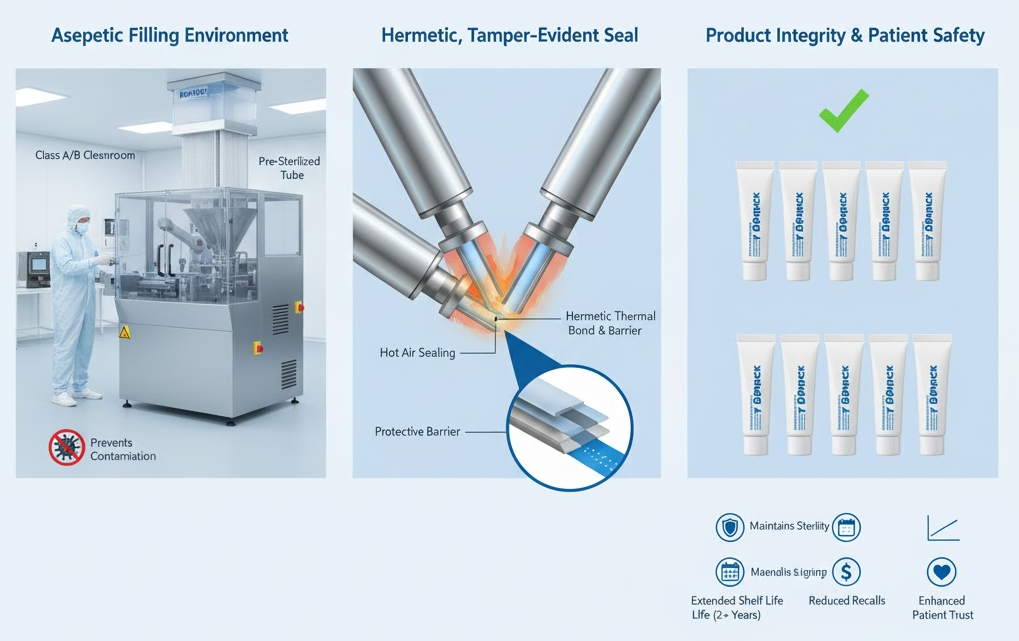
Each sealing method is selected based on tube material — laminate, plastic, or aluminum — to optimize heat distribution and joint integrity. Manufacturers like King Pack design their sealing stations to maintain uniform temperature control and clean airflow to meet aseptic production requirements.
Tubes are often exposed to variable humidity and mechanical stress during distribution. A robust seal prevents breaches in the packaging that could compromise internal sterility. In this sense, sealing technology not only completes the aseptic process but also extends its protective effect long after the filling operation.
Recommended Reading: How does the ointment filling machine work? – King Pack Machinery
Mechanical Integrity and Tamper Evidence
Mechanical integrity is essential for maintaining product quality from production to end use. Ophthalmic ointment tubes go through multiple handling stages — from filling lines and cartoning equipment to palletization and shipping. These steps subject the tubes to pressure, torsion, and vibration. A strong seal ensures that the tube does not deform, leak, or delaminate under these conditions.

KPGFW-60D drop filling and sealing machine – King Pack Machinery
A reliable seal also plays a central role in consumer confidence. It functions as the first line of tamper evidence, allowing users and pharmacists to confirm that a product has not been opened or altered. The visible, intact seal reassures patients that their medication is uncontaminated and safe for direct ocular application.
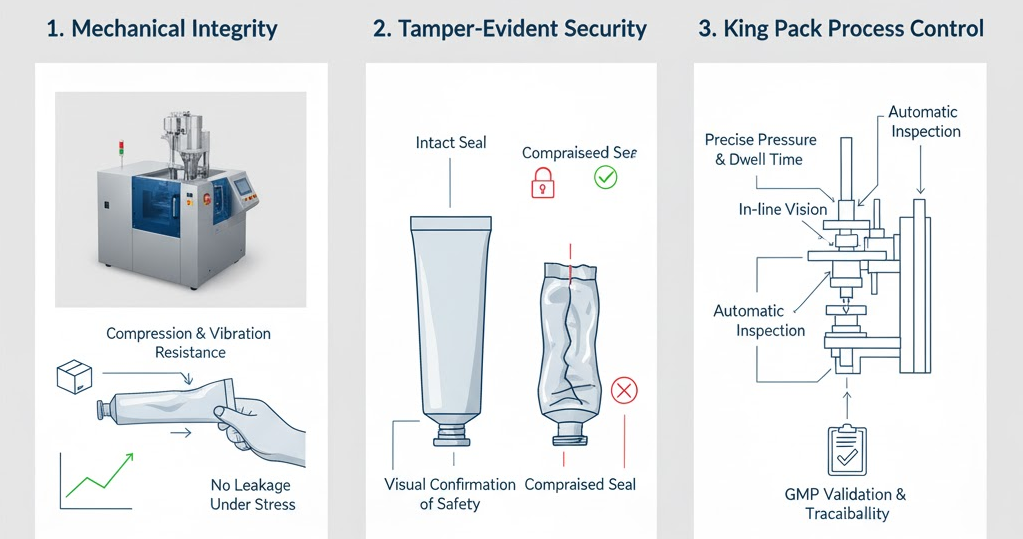
For medical products where even minor tampering can pose serious risks, the visual confirmation provided by the seal adds a crucial layer of safety and trust. To achieve this, King Pack’s tube filling and sealing machines incorporate precise control systems that regulate sealing pressure and dwell time. This ensures consistent seal quality across high-speed operations.
When combined with in-line inspection units, manufacturers can automatically verify seal integrity, detect defects, and reject non-conforming units. Such process control aligns with GMP validation protocols and helps pharmaceutical companies maintain traceable quality assurance from batch to batch.
Recommended Reading: What are the advantages of tube filling machine? – King Pack Machinery
Maintaining Viscosity, Stability, and Drug Efficacy Over Time
The seal also preserves the internal formulation’s physical and chemical stability. Ophthalmic ointments often rely on petrolatum or mineral oil bases that can harden, separate, or lose consistency if exposed to air or moisture. A secure, airtight seal prevents these external factors from disrupting the internal balance.
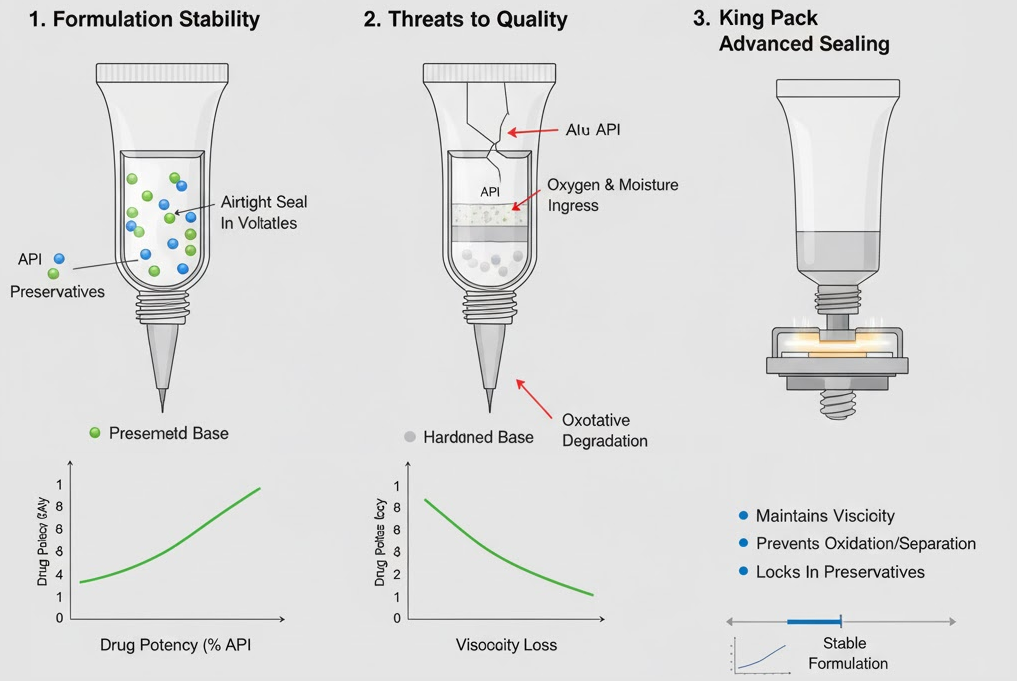
This protection maintains the desired viscosity, which is vital for accurate dosing and comfort during application. Many ophthalmic preparations contain low concentrations of preservatives to reduce irritation. Over time, volatile components can evaporate through a weak seal, reducing preservative levels below their effective threshold.
A properly executed seal locks in these sensitive compounds, maintaining microbial protection and product performance throughout storage. Without it, even a stable formulation can become unsafe for use. The same principle applies to multi-active formulations. Some modern ophthalmic ointments contain a combination of APIs, each with distinct stability requirements.
The seal prevents oxidative degradation, phase separation, and potency loss over time. By maintaining a controlled microenvironment inside the tube, sealing ensures the formulation remains therapeutically active up to its expiration date — a direct reflection of both manufacturing quality and brand reliability.
Recommended Reading: How Does a Tube Filling Machine Work? – King Pack Machinery
Tube Sealing Technologies Used in Ophthalmic Production
Hot Air Sealing for Plastic and Laminate Tubes
Hot air sealing directs precisely controlled heated air at the tube’s internal surfaces, softening the thermoplastic layers until they fuse under applied pressure. This method works well for polyethylene, polypropylene, and multi-layer laminate tubes commonly used in pharmaceutical applications.
Temperature control proves critical—insufficient heat produces weak seals prone to failure, while excessive heat can deform the tube or degrade heat-sensitive ointment residues near the seal area. Advanced tube filling and sealing systems incorporate servo-controlled hot air modules that adjust parameters in real-time based on tube material and ambient conditions.
Aluminum Tube Crimping for Sterile Ointments
Aluminum tubes require mechanical crimping rather than heat fusion. The crimping die folds the tube end multiple times, creating overlapping layers that trap any residual ointment and form a gas-tight seal. This cold-forming process avoids the heat exposure that might affect thermally sensitive APIs.
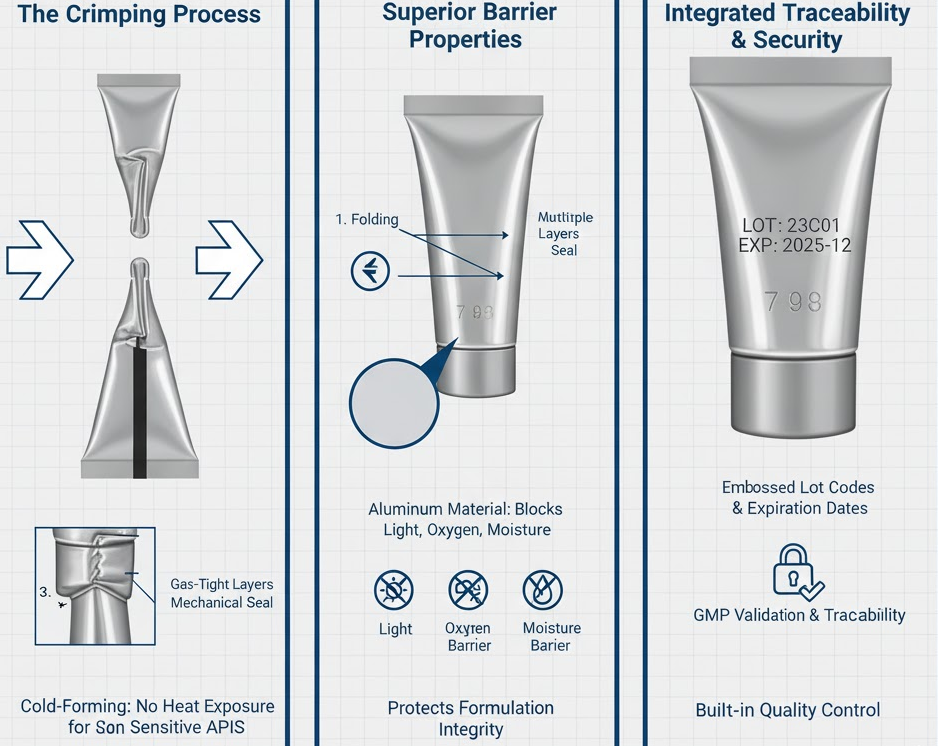
Aluminum tubes offer superior barrier properties against light, oxygen, and moisture compared to plastic alternatives, making them preferred for light-sensitive ophthalmic formulations. The crimping process can also emboss lot codes and expiration dates directly into the seal, eliminating the need for separate printing operations.
Recommended Reading: The Differences Between Homogenization and Emulsification – King Pack Machinery
Ultrasonic Sealing and its Applications in Aseptic Lines
Ultrasonic sealing employs high-frequency mechanical vibrations to generate localized heating at the molecular level within thermoplastic materials. The process occurs rapidly—typically in less than one second—minimizing heat transfer to the product.
This speed advantage makes ultrasonic sealing attractive for high-throughput aseptic lines where maintaining environmental classification during sealing operations presents challenges. The technology works particularly well with laminate tubes, creating consistent, strong seals without the thermal exposure required by hot air methods.
Integration with Aseptic Manufacturing Lines
Compatibility with Filling–Sealing–Coding Lines
Modern pharmaceutical manufacturers demand integrated systems where filling, sealing, and coding occur in a continuous, validated process. Tube filling machines from manufacturers like King Pack incorporate this integration, featuring synchronized rotary or linear transport systems that move tubes through filling stations, seal stations, and coding stations without manual intervention.
PLC control systems coordinate all operations, tracking each tube’s position and automatically rejecting any unit that fails to meet specified parameters. These integrated lines can handle production speeds from 30 tubes per minute for smaller operations to over 100 tubes per minute for high-volume manufacturers.
Minimizing Human Intervention to Reduce Contamination
Aseptic processing principles require minimizing human contact with sterile products and their primary packaging. Automated tube feeding systems eliminate the need for operators to manually load tubes into filling stations. Photoelectric sensors detect tube presence and orientation, while servo motors precisely position tubes for filling and sealing.
Some advanced systems incorporate automated tube cleaning using compressed air to remove particulates before filling—a feature particularly relevant for plastic tubes that may generate electrostatic charges attracting dust. The enclosed design of pharmaceutical-grade tube fillers, constructed from stainless steel with minimal crevices, supports effective cleaning and sanitization between production runs.
Recommended Reading: Emulsifier Machine: Types of Vacuum Emulsifying Mixers & Applications – King Pack Machinery
Traceability, Validation & In-Line Quality Inspection
Regulatory compliance requires comprehensive documentation of sealing parameters for each production batch. Modern filling machines record critical process parameters—seal temperature, dwell time, pressure, and seal integrity test results—creating an electronic batch record that supports product release decisions.
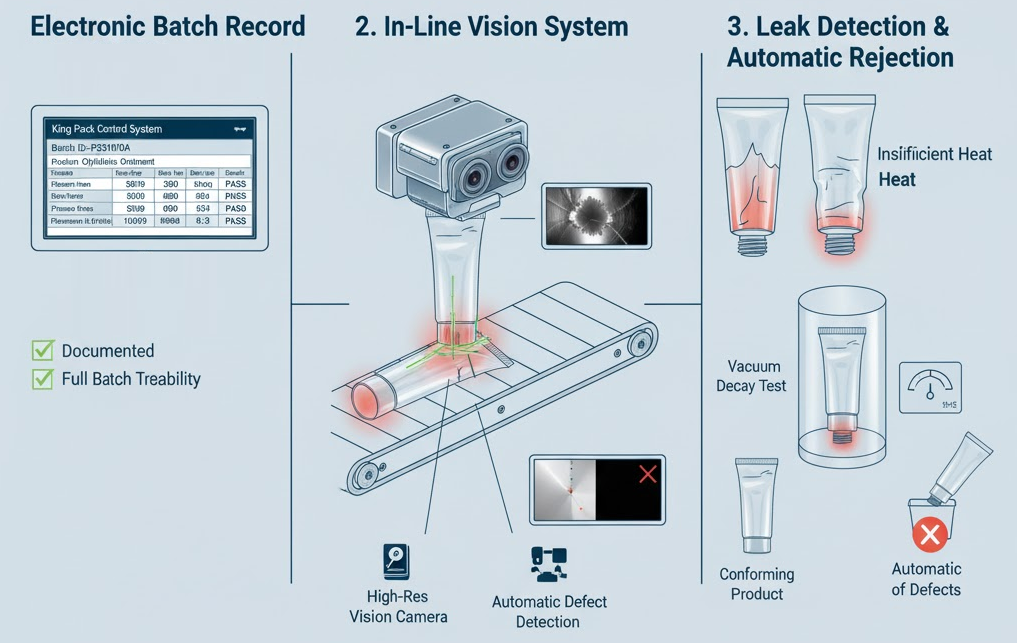
In-line vision systems inspect seal formation, verifying uniform seal width and detecting gross defects. Some manufacturers incorporate leak detection stations that test random samples from each batch using methods like vacuum decay or pressure differential measurement. These quality systems provide real-time feedback, allowing operators to adjust parameters before producing significant quantities of non-conforming product.
Why Proper Tube Sealing Impacts Patient Safety and Brand Trust
Preventing Recontamination During Storage and Use
Multi-dose eye drop filling machines and ointment tube filling machines must maintain tight container closing to preserve sterility across multiple uses. Once opened, plastic bottles or tubes are exposed to air and handling, increasing the chance of contamination. A strong initial seal formed under controlled CIP/SIP conditions prevents microbial entry before the first dose is dispensed.
If the seal is weak, baseline contamination can develop, overpowering preservatives and creating infection risks. Reliable heat sealing or ultrasonic sealing technology ensures airtight protection, while systems equipped with Torque monitoring and photo-electricity sensors confirm each closure’s integrity. Properly sealed containers also withstand fluctuating storage conditions in bathrooms or medicine cabinets, where temperature and humidity vary significantly.
Reducing Complaints, Recalls, and Legal Risk
Failures in tube or nozzle filling machine closures can lead to leakage, hardening of the ointment, or visible deformation during dispensing. These issues trigger costly investigations, production holds, and potential product recalls. The use of 304 stainless steel construction and PLC with touch screen control system helps maintain filling precision and hygiene, reducing the risk of such complaints.
Validated sealing lines featuring volumetric dosing systems, safety protection devices, and Gas flushing reduce contamination risks and extend shelf life. If contamination or closure failure occurs, manufacturers face recall costs, legal challenges, and brand damage. Documented compliance with GMP Ophthalmic Filling Room requirements strengthens a company’s ability to demonstrate quality control during audits or legal reviews.
Protecting Sterile Class Reputation in Ophthalmic Markets
In sterile production, reputation depends on proven container integrity and aseptic process control. Facilities using Eye Drop Filling Machines, Automatic Ointment Filling Machines, and vertical laminar flow hoods maintain Class A/B cleanliness while handling sensitive ophthalmic formulations.
Companies that integrate peristaltic pumps, volumetric dosing systems, and quickconnect technology achieve consistent dosing with minimal human contact—key to meeting GMP and ISO standards. Conversely, poor sealing or contamination events invite regulatory scrutiny, damaging trust among ophthalmologists, pharmacists, and healthcare institutions. Reliable capping machines and sorting units help uphold brand credibility, ensuring every package leaves the line sterile, sealed, and compliant.
Conclusion
Tube sealing in ophthalmic ointment production is more than just packaging—it’s the final step that safeguards sterility, stability, and product safety. A proper seal must resist mechanical stress, block contaminants, show tamper evidence, and protect the active ingredients through the product’s shelf life.
To meet these standards, manufacturers need sealing technologies matched to tube materials, supported by strong quality systems and validated under GMP. Choosing between hot air sealing for plastic tubes, crimping for aluminum, or ultrasonic sealing depends on formulation, batch size, and market regulations.
Contact us to learn how King Pack’s validated tube filling and sealing systems deliver the precision, traceability, and control needed for sterile ophthalmic production.

