
What Are Vial Filling Machines & Their Importance
Core Functionality and Key Components (Conveyor, Filling Heads, Stoppering)
Vial filling machines automate pharmaceutical product dispensing into sterile glass containers. These systems consist of several integrated components working in synchronized precision.
Each element plays a critical role in maintaining product quality. The conveyor system transports vials through each processing stage. Filling heads dispense exact volumes into containers. Stoppering mechanisms place rubber closures immediately after filling completes.
Dose accuracy affects patient safety directly. USP General Chapter 697 requires each injection container contains sufficient excess volume to allow withdrawal of the labeled drug quantity. Manufacturers must balance minimal waste against extractable volume requirements.
Two major challenges during filling are fill weight accuracy/precision and potential impact on product quality. Non-optimized processes lead to high reject rates. They also risk contamination during visual inspection.
Throughput determines production economics. Over 5 billion injectable doses were produced globally in 2023. High-speed pharmaceutical vial filling machine systems meet this enormous demand while maintaining quality standards.
Recommended Reading: What is a Vial Filling Machine? – King Pack Machinery
Types of Vial Filling Machines by Product/Formulation
Liquid Vial Filling Machines – For Injectables, Vaccines, Liquids

Liquid vial filling machines form the backbone of sterile injectable production. In 2023, the pharmaceutical sector contributed nearly 40% of total liquid vial filling machine market revenue, driven by growing vaccine and biologic manufacturing.
These machines fill vials with solutions such as vaccines, antibiotics, and biologics under aseptic conditions. Accuracy and sterility are non-negotiable—especially when working with high-value or sensitive ingredients.
King Pack’s liquid vial filling machines are designed to meet these exact standards, providing reliable operation across small, medium, and large-scale production lines.
Each filling method supports specific product and process needs. Choosing the right one depends on product viscosity, sterility requirements, and production speed.
| Filling Method | Ideal For | Key Advantage | Notes |
| Piston Pump Filling | Most aqueous and viscous liquids | High volumetric accuracy (±0.5%) | Widely used for vaccines, injectables |
| Peristaltic Pump Filling | Sensitive biologics, sterile solutions | No product contact with pump components | Easy to clean and maintain sterility |
| Time-Pressure Filling | Medium-to-high viscosity products | Flexible for varying viscosities | Suitable for syrups and suspensions |
Key Benefits of King Pack Liquid Filling Systems
- High fill precision: Consistent volume control even for micro-dose vials.
- Fully enclosed operation: Maintains aseptic environment and product sterility.
- Modular design: Compatible with stoppering, capping, and inspection modules.
- Quick changeover: Supports multiple vial sizes without long setup times.
- Automated vision inspection: Detects fill volume deviations and rejects non-compliant units.
With these features, King Pack’s systems align with GMP standards and meet regulatory expectations for sterile manufacturing. They provide a balance of precision, flexibility, and scalability—ideal for injectables, vaccines, and other liquid pharmaceutical products.
Powder Vial Filling Machines – Dry Formulations, Lyophilized Products
Powder vial filling machines play a crucial role in handling dry formulations, especially antibiotics, hormones, and lyophilized biologics that degrade easily in liquid form. These systems support the production of stable, moisture-free pharmaceuticals that can be reconstituted before injection.

The global powder vial filling machine market is expanding rapidly, projected to grow at a 7.8% CAGR through 2028, driven by automation, the rising use of freeze-dried drugs, and the growing demand for long-shelf-life formulations. For manufacturers, this means increased focus on precision dosing, gentle handling, and contamination-free operation.
Powders vary widely in density, flowability, and particle size, requiring flexible filling mechanisms. King Pack offers multiple systems to match these characteristics while maintaining dosing accuracy.
| Filling Method | Ideal For | Key Advantage | Notes |
| Auger Dosing | Free-flowing or semi-free-flow powders | Consistent fill weight via screw rotation | Ideal for antibiotics and enzyme powders |
| Vacuum Filling | Fine or cohesive powders | Accurate fill through pressure differential | Minimizes dust and product loss |
| Dual-Head Systems | High-speed or large-batch production | Parallel operation increases throughput | Suitable for industrial-scale filling |
Lyophilized products need special treatment after filling. Partially stoppered vials enter freeze-drying chambers where moisture is removed under vacuum. Once lyophilization completes, the system reseats the stoppers and proceeds to capping and sealing. King Pack integrates these steps seamlessly within automated production lines.
Key Benefits of King Pack Powder Filling Systems
- High dosing precision: Achieves fill weight tolerance as tight as ±1%.
- Closed filling environment: Protects sensitive powders from ambient moisture.
- Modular integration: Compatible with vial washing, stoppering, and sealing units.
- Gentle powder handling: Maintains product integrity without dust formation.
- Easy cleaning and changeover: Reduces downtime between batches.
King Pack’s powder vial filling solutions provide flexibility for a wide range of dry products—combining accuracy, sterility, and reliability for modern pharmaceutical production.
Injectable Liquid Filling Machines – High-Precision, Regulatory Focus
Injectable formulations are among the most sensitive and tightly regulated in the pharmaceutical sector. Over 70% of new FDA-approved drugs in 2022 and 2023 were biologics or injectables, reflecting a global shift toward advanced therapies that demand strict sterility and micro-dosing precision.
These products often include vaccines, monoclonal antibodies, and ophthalmic treatments where even minor inconsistencies can affect safety or efficacy.
Injectable production operates under Good Manufacturing Practice (GMP) guidelines, requiring validated processes and complete traceability. Every parameter—temperature, humidity, pressure, and fill volume—must be monitored and recorded.
King Pack’s injectable vial filling machines are engineered for 21 CFR Part 11 compliance, supporting data integrity and electronic batch records essential for regulatory audits.
| Compliance Feature | Purpose | King Pack Implementation |
| GMP-Compliant Design | Prevents contamination and mix-ups | Smooth stainless-steel surfaces, minimal dead zones |
| Validation Support (IQ/OQ/PQ) | Verifies machine qualification | Comprehensive documentation package provided |
| Data Integrity (21 CFR Part 11) | Tracks parameters for each batch | Secure electronic records and audit trails |
| HEPA-Filtered Laminar Flow | Maintains ISO Class 5 environment | Controlled air zones during filling and stoppering |
Many injectables, such as intravitreal injections, require extremely small fill volumes ranging from 10 to 100 µL. Maintaining accuracy at these scales is technically demanding.
King Pack machines use servo-driven piston and peristaltic pumps to deliver consistent micro-dosing performance. Real-time feedback systems detect pressure or flow variations instantly, adjusting during operation to maintain target volume.
Key Benefits of King Pack Injectable Filling Systems
- Ultra-high accuracy: Tolerance within ±0.5%, even for micro volumes.
- Sterile automation: Filling, stoppering, and capping occur under Grade A conditions.
- Flexible configuration: Supports both bulk and ready-to-use vials or prefilled syringes.
- Validation ready: Includes calibration, FAT/SAT support, and regulatory documentation.
- Integrated inspection: Vision systems verify fill level and stopper position for 100% of vials.
King Pack’s injectable vial filling machines combine precision dosing, GMP compliance, and advanced automation—helping manufacturers meet today’s regulatory expectations while protecting product integrity from start to finish.
Click here to check our products
Key Ancillary Machines & Line Components
Vial Capping and Sealing Machines – Ensuring Closure Integrity
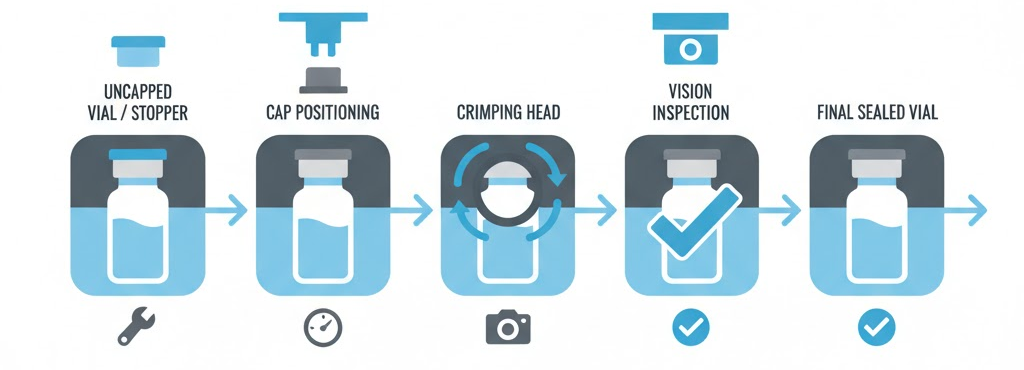
Aluminum caps secure rubber stoppers permanently. Crimping mechanisms fold cap edges around vial necks. Proper sealing protects contents throughout shelf life.
Torque control prevents under or over-crimping. Vision systems inspect crimp quality automatically. Reject mechanisms remove defective closures immediately. King Pack’s vial capping and sealing machine solutions integrate seamlessly with filling equipment.
Multiple cap styles accommodate different applications. Flip-off caps suit clinical settings. Tear-off caps prevent reuse. Color-coding aids product identification. Equipment handles various formats through quick-change tooling.
Recommended Reading: A Detailed Guide to Vial Filling Machine Types – Principles and Selection | King Pack – King Pack Machinery
Vial Washing Machines – Pre-filling Sterility Preparation
Vials arrive with manufacturing residues. Washing removes particles before filling begins. Water for Injection (WFI) serves as the cleaning medium.
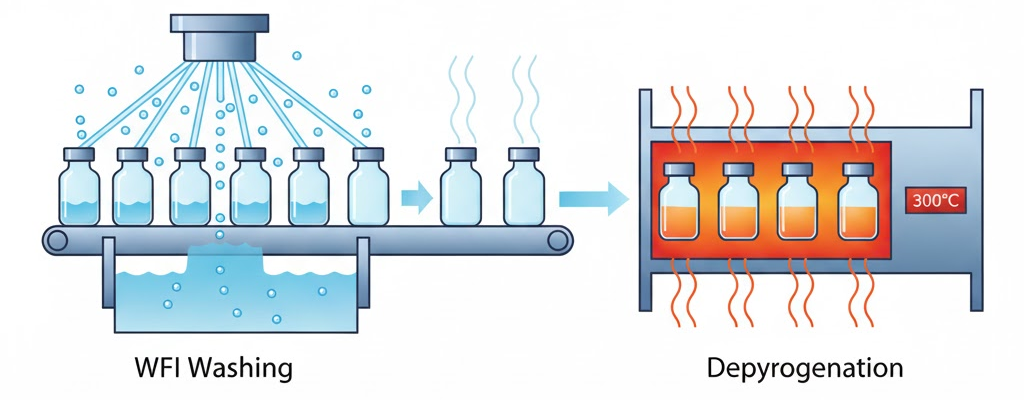
Depyrogenation follows washing. Dry heat eliminates endotoxins at 250-350°C. Installation of automated filling lines grew over 30% from 2021 to 2023. This surge reflects increased focus on sterile processing capabilities.
King Pack’s systems prepare containers to pharmaceutical standards. Validated processes confirm cleanliness. This prevents contamination before products enter vials.
Recommended Reading: Vial Filling Machines and Processing Solutions – King Pack Aseptic Technology
Full Vial Filling Lines – Integration of Filling, Stoppering, Capping, Labelling
Complete lines automate entire workflows. Products flow from washing through final inspection. Over 1,200 pharmaceutical facilities globally adopted RABS or isolators. This drives demand for compatible integrated systems.
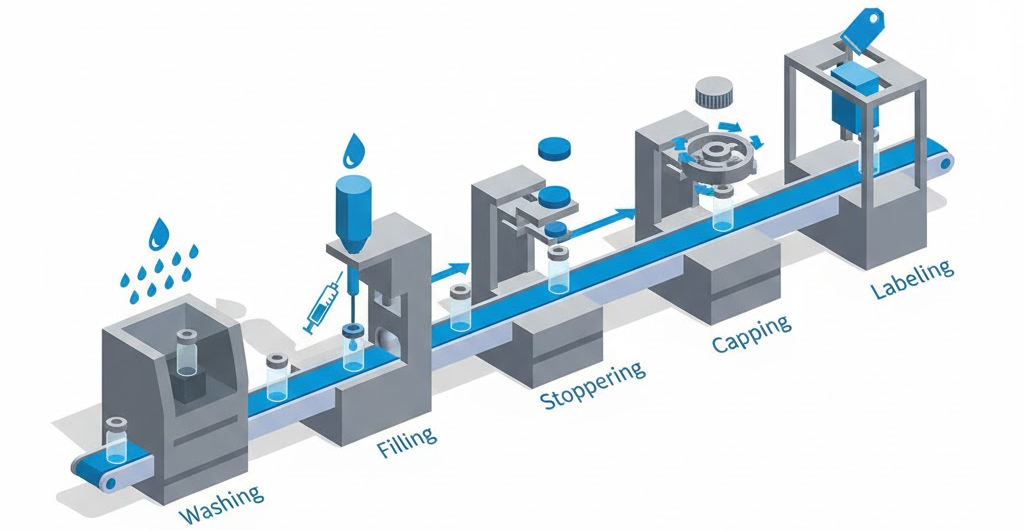
King Pack’s sterile vial filling line solutions include all necessary components. Washing, filling, stoppering, capping, and labeling operate in coordinated sequence. Single-source responsibility simplifies validation and support.
Integration reduces contamination risks. Products spend less time exposed to environments. Automated transfers eliminate manual handling. This maintains sterility throughout processing.
Working Principles & Customization Options
Number of Filling Heads (2-20 etc.) & Their Impact on Speed
Filling head count determines throughput capacity. Single-head machines suit research applications. Multi-head systems handle commercial production volumes.
Two-head configurations provide basic automation. Six to twelve heads suit medium production. Twenty-head systems achieve maximum speeds. King Pack customizes head counts to match customer requirements.
Speed increases proportionally with heads. A four-head machine at 100 vials per minute per head produces 400 total. Synchronization maintains consistent quality across all positions.
Filling Principles: Volumetric, Time-Pressure, etc.
Volumetric piston filling delivers exact displacement. Each stroke provides repeatable volumes. Accuracy reaches ±0.5% across normal ranges. This suits most pharmaceutical applications.
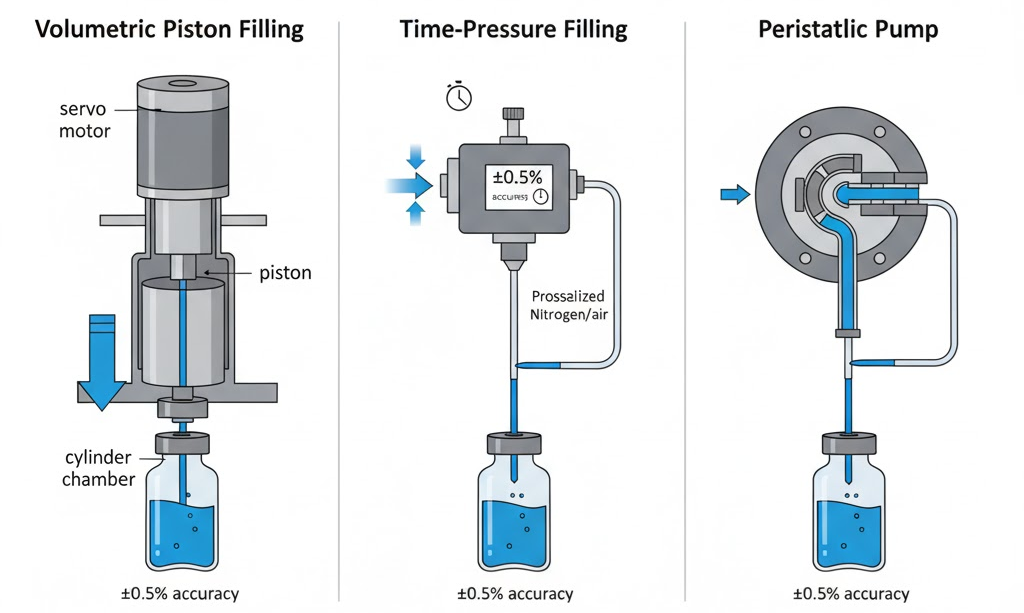
Time-pressure systems meter by controlling valve duration. Servo valves open for calculated intervals. Fill accuracy depends on consistent product properties. This accommodates formulations with varying viscosity.
Peristaltic pumps use rotating rollers compressing flexible tubing. Product never contacts pump components. This simplifies changeover between formulations. Biologics benefit from gentle, non-shearing flow.
Materials, Nozzles, Changeover Flexibility
All product-contact surfaces use 316L stainless steel. This grade resists corrosion from pharmaceutical ingredients. Polished finishes prevent bacterial adhesion. King Pack follows pharmaceutical construction standards.
Filling nozzles require specific geometries. Bottom-up filling reduces foam formation. Nozzles enter bottles and rise as liquid fills. Anti-drip valves prevent mess between cycles.
Quick-change capabilities reduce downtime. Tool-free adjustments accommodate various vial sizes. Recipe management stores parameters for multiple products. Operators switch formulations through touchscreen controls.
How to Choose the Right King Pack Vial Filling Machine
Match Machine Type to Your Product (Liquid / Powder / Injectable)
Every product has unique handling needs. Liquids, powders, and injectables each require different filling systems and dosing methods.
For example, automatic volumetric multi head vial liquid filling machines use precise dosing pumps and servo-driven accuracy to handle both thin and viscous formulations. For injectable powder filling, specialized dosing mechanisms and ceramic dosing syringes maintain consistent fills while preventing product loss.
King Pack engineers study each formulation’s viscosity, temperature range, and sensitivity before recommending equipment. High-viscosity liquids may benefit from dynamic motion filling or temperature control, while delicate biologicals often need gentle aseptic filling machines with laminar flow protection. For powders, moisture level and particle size affect flow, so equipment selection focuses on stability and dosing precision.
Consider Production Rate and Automation Level
Production needs vary across research labs, pilot plants, and large-scale healthcare industries. Small-scale facilities might rely on compact electronic filling machines or semi-automatic units that balance cost and flexibility. These systems usually include manual vial loading but automate the filling stations and stoppering stations to improve productivity.
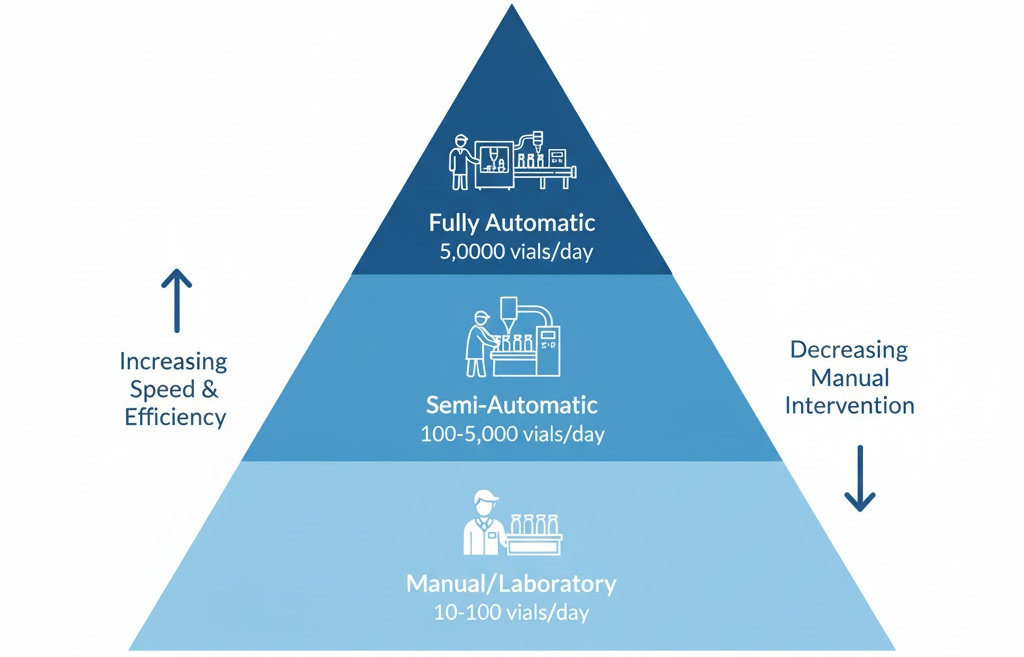
When higher throughput is required, filling and closing lines or the CR Series deliver faster performance with complete automation. Fully automatic setups integrate stoppering machines, Labeling machines, and even Aseptic Isolators to maintain sterility.
Optional modules like Nitrogen Flushing or eccentric pre-gassing can be added to protect oxygen-sensitive products. King Pack’s use of servo technology and star wheel positioning systems provides reliable pumps output and smooth handling for vials in trays or continuous lines.
Regulatory Compliance (Sterility, GMP), Materials, Cleanability
Stringent regulations regarding drug safety and quality drive pharmaceutical vial filling machine adoption. Equipment must support GMP compliance. King Pack designs meet international pharmaceutical standards.
Validation documentation proves performance. Installation Qualification (IQ) confirms proper setup. Operational Qualification (OQ) verifies functionality. Performance Qualification (PQ) demonstrates process capability.
Cleanability affects compliance and efficiency. CIP (Clean-In-Place) systems automate sanitation. Sterilize-in-place capability uses steam for deep cleaning. Quick-disconnect fittings simplify maintenance access.
Recommended Reading: How to Choose the Right Vial Filling Machine for Your Business – King Pack Machinery
King Pack Value Proposition
Competitive Pricing with High Build Quality
King Pack delivers pharmaceutical-grade equipment at accessible prices. European engineering standards guide all designs. Quality materials and components provide long-term reliability.
Investment analysis considers total ownership costs. Energy-efficient components reduce operating expenses. Reliable operation minimizes costly downtime. Return on investment accelerates through improved productivity.
High initial cost of acquiring advanced filling machines challenges small manufacturers. King Pack addresses this through flexible configurations. Customers purchase capabilities matching current needs with expansion options.
Flexibility and Customization for Different Pharma Requirements
Every facility faces unique challenges. Floor space, production volume, and product characteristics vary widely. King Pack’s vial filling machines adapt to specific requirements through modular design.
Format flexibility accommodates various vial sizes. Quick-change tooling reduces downtime during product switches. Recipe storage remembers settings for each formulation. This simplifies operations in multi-product facilities.
Integration capability connects with existing equipment. Upstream preparation feeds filling machines. Downstream inspection and packaging receive finished vials. King Pack engineers coordinate all interfaces.
Global Support, Spare Parts, After-Sales Service
Equipment purchases begin long-term relationships. King Pack supports customers throughout machine lifecycles. Technical assistance available 24/7 addresses urgent issues promptly.
Spare parts ship quickly from regional warehouses. Critical components stock locally for fastest delivery. This minimizes production downtime from unexpected failures. Parts availability extends years beyond initial purchase.
Training programs prepare operators and maintenance staff. Hands-on instruction at customer facilities provides practical experience. Ongoing education keeps teams current on best practices.
Conclusion
King Pack delivers comprehensive vial filling and processing solutions. Contact King Pack today for quotes, technical specs, or demo. Our experienced team evaluates production requirements and recommends optimal configurations.
You can also request a consultation to discover how advanced vial filling technology can improve your pharmaceutical manufacturing efficiency, and how we can help you in achieving consistent quality, faster turnaround, and full GMP compliance. We don’t just supply machines; we help you build a reliable production process that reduces waste, maximizes uptime, and safeguards product integrity.

