Blister packaging plays a central role in pharmaceutical drug delivery. At its core, a blister pack consists of a contoured cavity that holds a tablet or capsule, sealed by a foil or film lid. This arrangement preserves dosage integrity and supports patient safety — every dose is individually secured until use.
In this guide, we’ll explain how blister packaging works, what materials and methods are used, why it matters, and how to select the right solution. We’ll also touch on how King Pack’s machinery can support your pharmaceutical blister‑packaging line.
Definition and Purpose of Blister Packs
What Constitutes a Blister Pack (Cavity + Lidding Film/Foil)
A blister pack is made of two main parts that work together to protect and present the product. The first is the formed cavity, or the “blister,” which holds each tablet, capsule, or lozenge in a shaped pocket. The second is the lidding material, which seals the cavities to keep each dose safe, clean, and tamper-evident. Both parts are designed for durability, product safety, and ease of use.
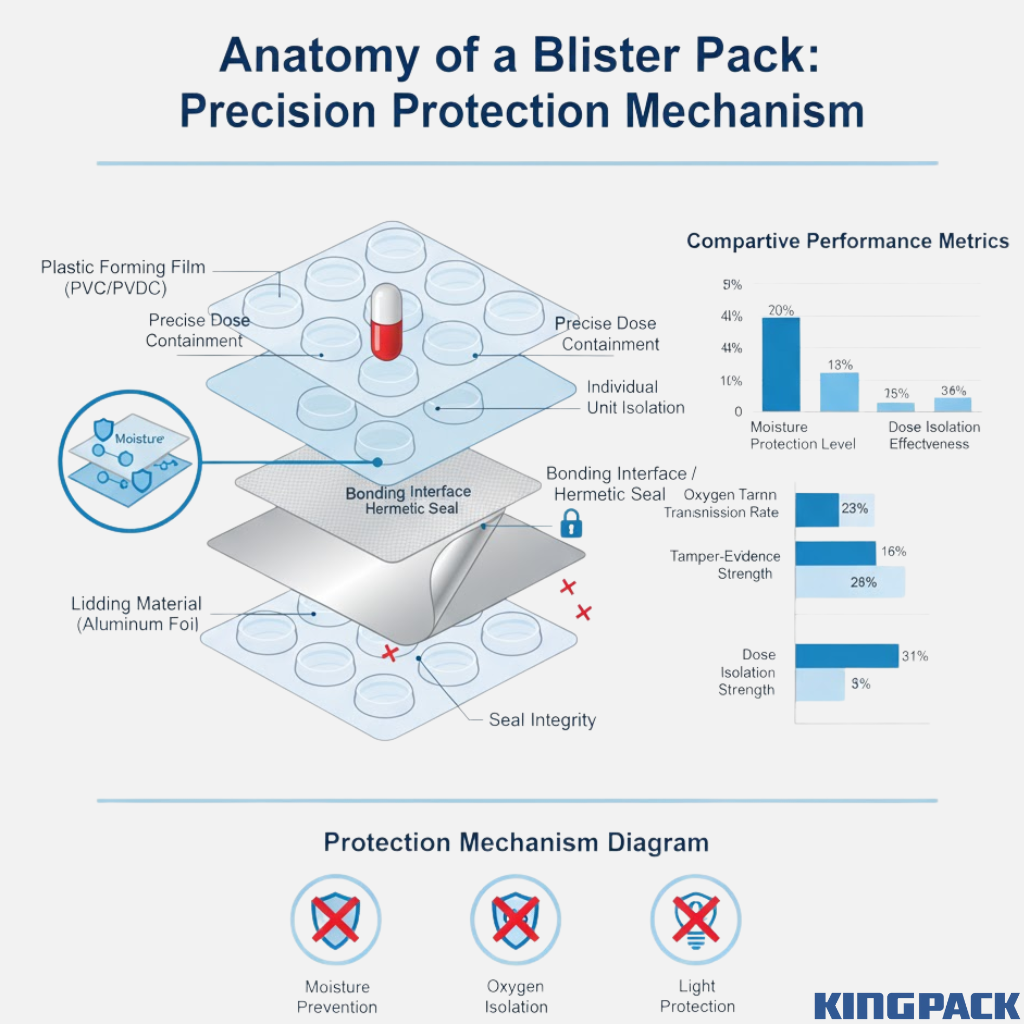
Main Components of a Blister Pack:
- Formed Cavity (Base Layer): Created through thermoforming or cold-forming of materials like PVC, PET, or aluminum. This layer provides the structure and houses individual doses securely.
- Lidding Material: Usually made of aluminum foil, but may also include plastic or paper-laminated films. It seals the formed base using heat or adhesive, creating an airtight and tamper-proof closure.
- Bonding Interface: The heat-sealed edge between cavity and lidding ensures each pocket remains isolated, preventing contamination or moisture entry.
Together, the cavity and lidding form a complete protective barrier that keeps pharmaceutical products stable throughout their shelf life.
The clear or semi-clear cavities allow easy inspection of tablets, while the printed foil offers space for branding, dosage instructions, and traceability information. Blister packs come in formats such as single-dose strips, multi-dose cards, and calendar-style packs, offering flexibility for different product and patient needs.
Recommended Reading: Efficiency and Sterility Breakthrough: King Pack S-Type CIP-SIP Pump In-Line Cleaning and Sterilization Process
Primary Role of Blister Packaging in Pharma – Protection, Dose Accuracy, Tamper Evidence
Blister packaging serves multiple critical functions in pharmaceutical distribution and use. Protection stands as the primary purpose—each sealed cavity shields its contents from moisture, oxygen, light, and physical damage. This isolated protection proves especially valuable for sensitive medications that degrade when exposed to humidity or air.
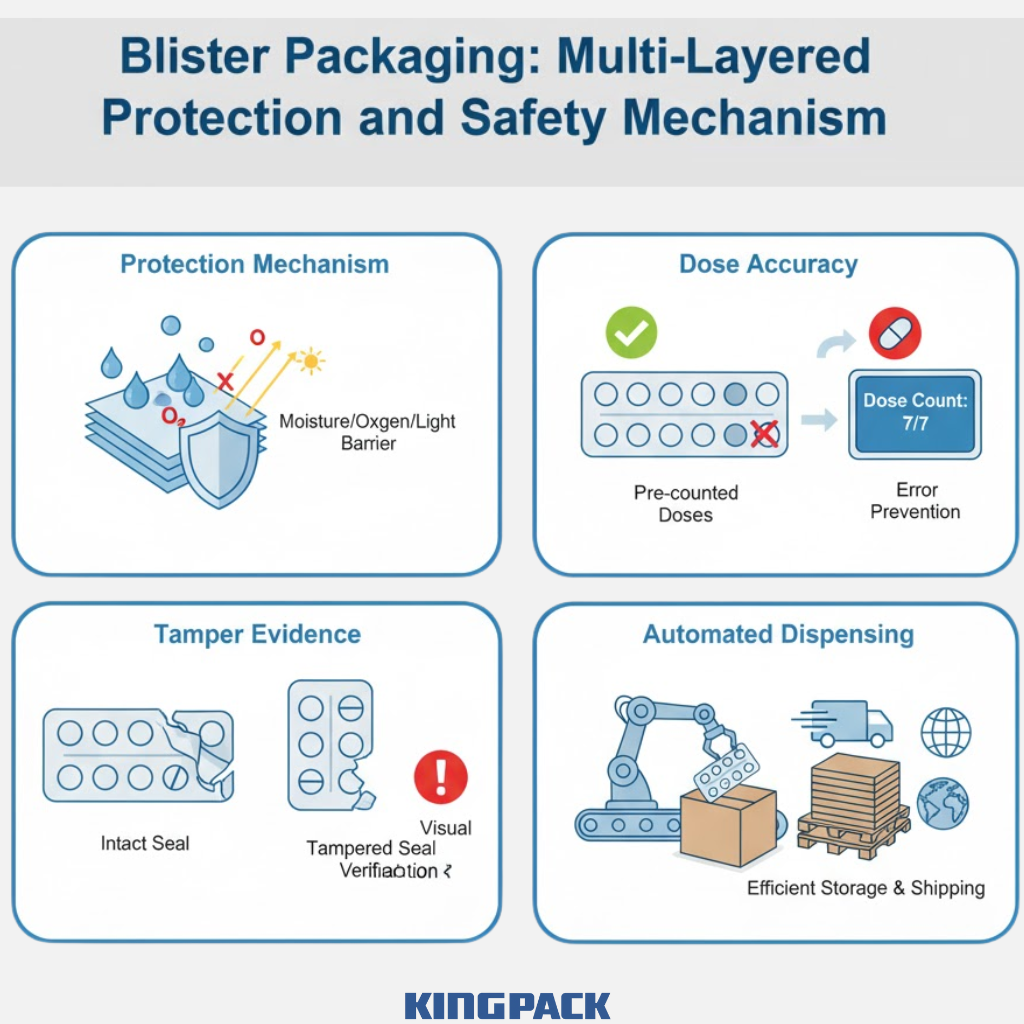
Dose accuracy represents another key benefit. Unlike bulk bottles where patients must count pills, blister packs provide pre-counted doses in individual compartments. This eliminates counting errors during dispensing and helps patients track medication intake. Missing blisters clearly indicate taken doses, supporting better adherence to prescribed regimens.
Tamper evidence has become increasingly important as counterfeit medications threaten patient safety worldwide. Blister packs provide visible evidence of tampering—accessing the medication requires breaking the seal or puncturing the cavity, actions that leave obvious marks. This feature helps pharmacists and patients verify product integrity before use.
Beyond these core functions, blister packs facilitate automated dispensing in pharmacies and hospitals. The standardized format allows robotic systems to handle and dispense medications efficiently. The flat profile enables compact storage and efficient shipping compared to bulky bottles.
Blister Packaging Materials & Construction
Material selection in blister packs is critical because it determines barrier protection, formability and cost. Common forming materials include PVC (polyvinyl chloride), PVDC coatings, and cold‑form aluminium foil systems.
The lid layers and seal types vary depending on protection needs and cost‑structure. And barrier properties — resistance to moisture, oxygen, light and chemical interaction — directly affect drug performance in storage.
Key materials & properties:
- PVC / PVDC films: cost‑effective thermoforming option, moderate barrier.
- Cold‑form Alu/Alu foil: highest barrier, ideal for moisture‑sensitive, long shelf‑life products.
- COC (cyclic olefin copolymer): newer plastic offering improved clarity and barrier for certain applications.
- Lidding materials: Aluminum foils or laminates that seal the cavity, sometimes with peel‑off or push‑through features.
- Barrier characteristics: A high‑barrier system shields the drug from moisture ingress, oxygen uptake and light exposure — critical for stability.
Choosing the right construction and seal type helps balance cost, protection and patient requirements — especially for complex drugs or extended‑shelf‑life products.
How Blister Packaging Machines Operate
Blister packaging lines transform rolls of plastic and aluminum foil into ready-to-use unit-dose packs. The process involves three main stages: forming cavities, filling tablets or capsules, and sealing them into individual pockets. Each step must align precisely to maintain packaging integrity and protect the medication from external factors.
King Pack’s automatic blister packaging machines use precise temperature, pressure, and motion control to deliver consistent blister quality. These systems integrate forming, sealing, and cutting in one continuous flow, which minimizes manual handling and reduces contamination risk.
Let’s explore how each stage works in a typical pharmaceutical blister packaging process.
Forming the Cavity – Thermoforming vs Cold‐Forming
Cavity formation is the first step in blister production. It defines the shape and depth of each pocket that holds the tablet or capsule. In thermoforming, the plastic film is heated until soft, then shaped using air pressure or mechanical molds. This method works well for standard medications with moderate barrier needs.
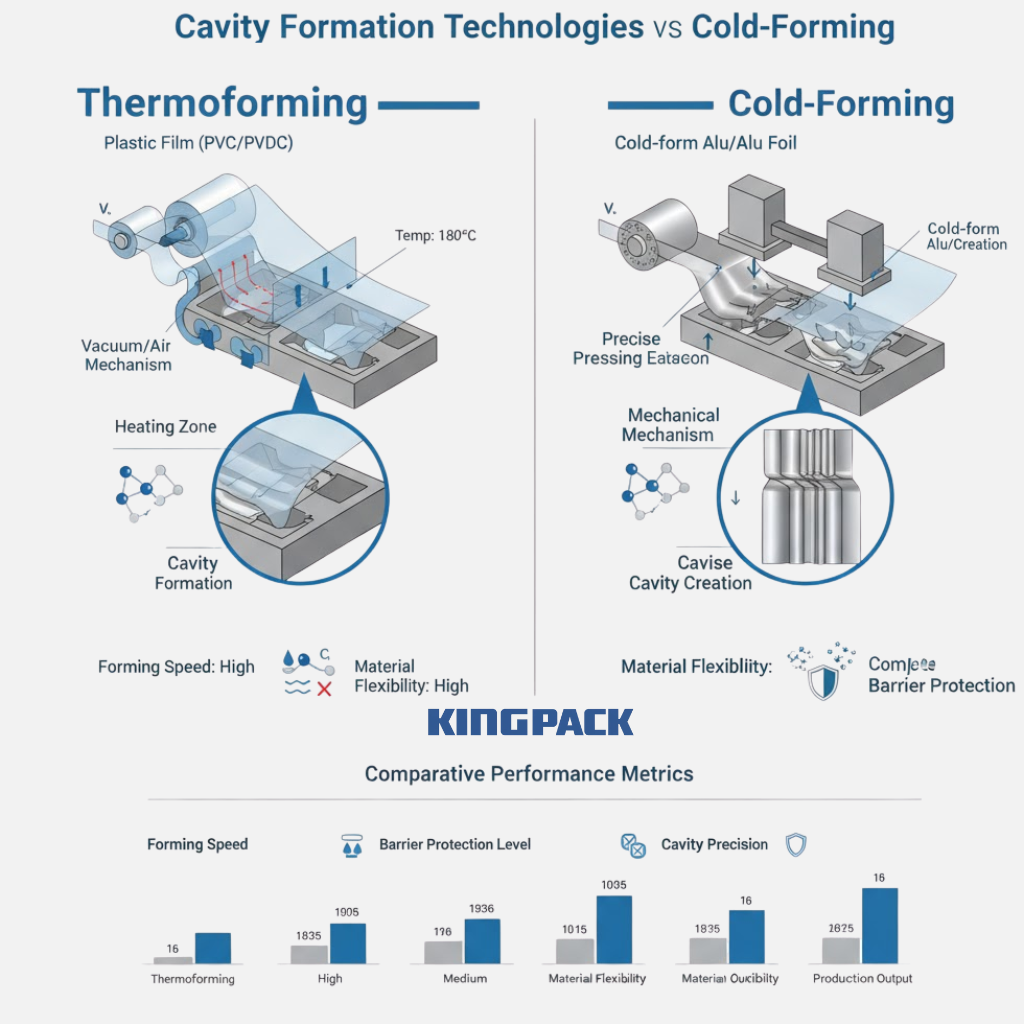
Cold-forming, on the other hand, relies on aluminum-based laminate films. The material is pressed into cavities without heat, providing excellent protection against moisture, oxygen, and light. This method is ideal for moisture-sensitive or long shelf-life unit-dose pharmaceuticals and specialist drugs.
King Pack’s automatic blister packaging machines combine precision cold-form materials and thermoform blisters in one packaging solution. Their alu-pvc-alu blister pack systems deliver smooth film handling and uniform forming depth, while ensuring durability and barrier performance.
Key Differences Between Thermoforming and Cold-Forming:
- Thermoforming uses PVC, PVDC, or COC films; cold-forming relies on aluminum laminates.
- Cold-forming offers superior barrier protection but operates at a slower speed.
- Thermoforming provides higher output and better tablet visibility.
- Cold-forming requires greater forming pressure and stronger molds for accurate cavity shaping.
Filling Tablets/Capsules into Blister Cavities
Once cavities are formed, the next step is accurate filling. Tablets or capsules are guided into the cavities through vibration channels, feeding plates, or dedicated pick-and-place systems. This stage demands precision — any misalignment can cause sealing defects or product loss.
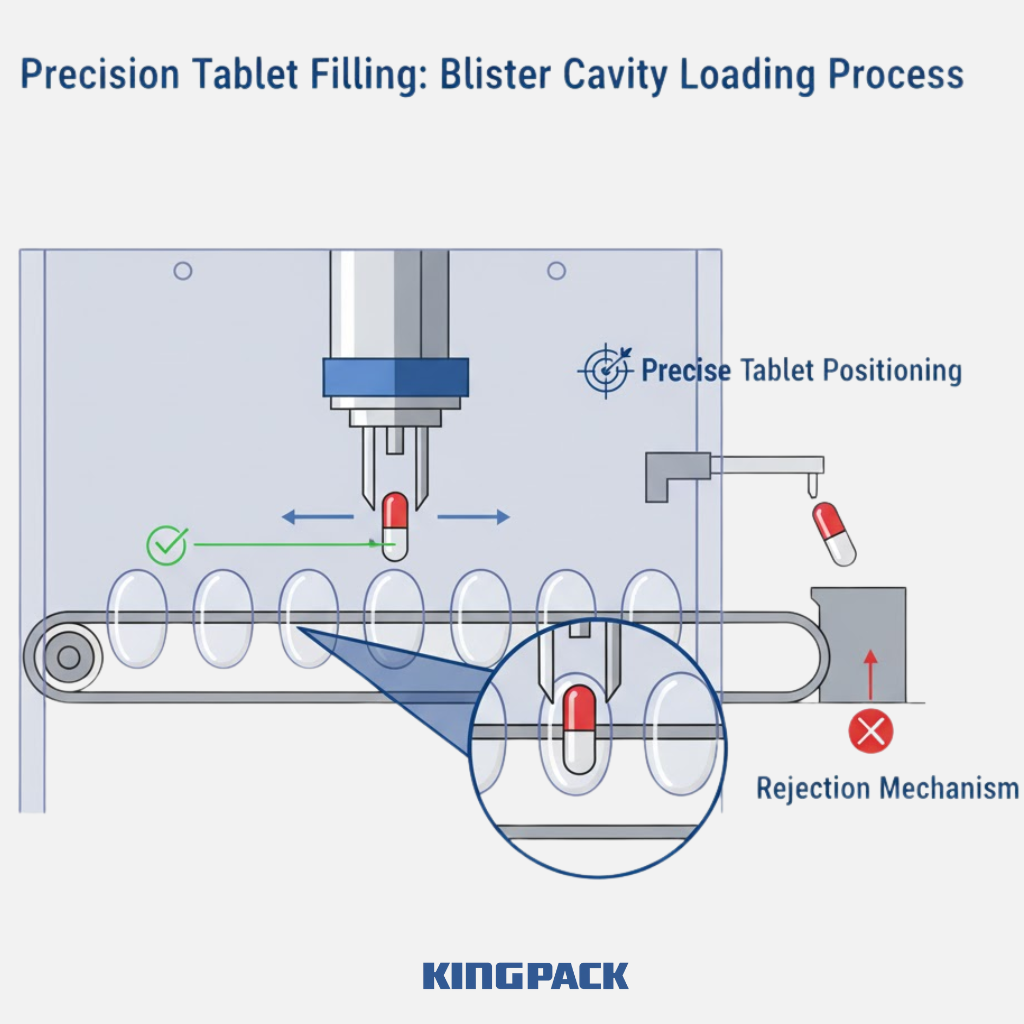
King Pack machines use optical and mechanical sensors to verify that each cavity receives one tablet or capsule. Unfilled or misaligned cavities are automatically rejected before sealing. The filling process can be adjusted to suit product size, shape, and weight, making it adaptable for both small-batch and high-speed operations.
Efficient filling ensures product safety and prevents cross-contamination. It also allows better tracking through batch coding and inline inspection systems.
Common filling systems used in blister machines:
- Vibratory feed systems for uniform flow
- Brush or suction-based positioning units
- Servo-driven pick-and-place systems for delicate products
- Inline cameras for fill verification
Sealing & Cutting / Punching into Blister Strips or Cards
After filling, the next step is sealing — where the lidding foil bonds to the formed web. Heat and pressure are applied to create a tight seal around each cavity. The temperature, dwell time, and sealing force are carefully controlled to avoid damaging heat-sensitive drugs.
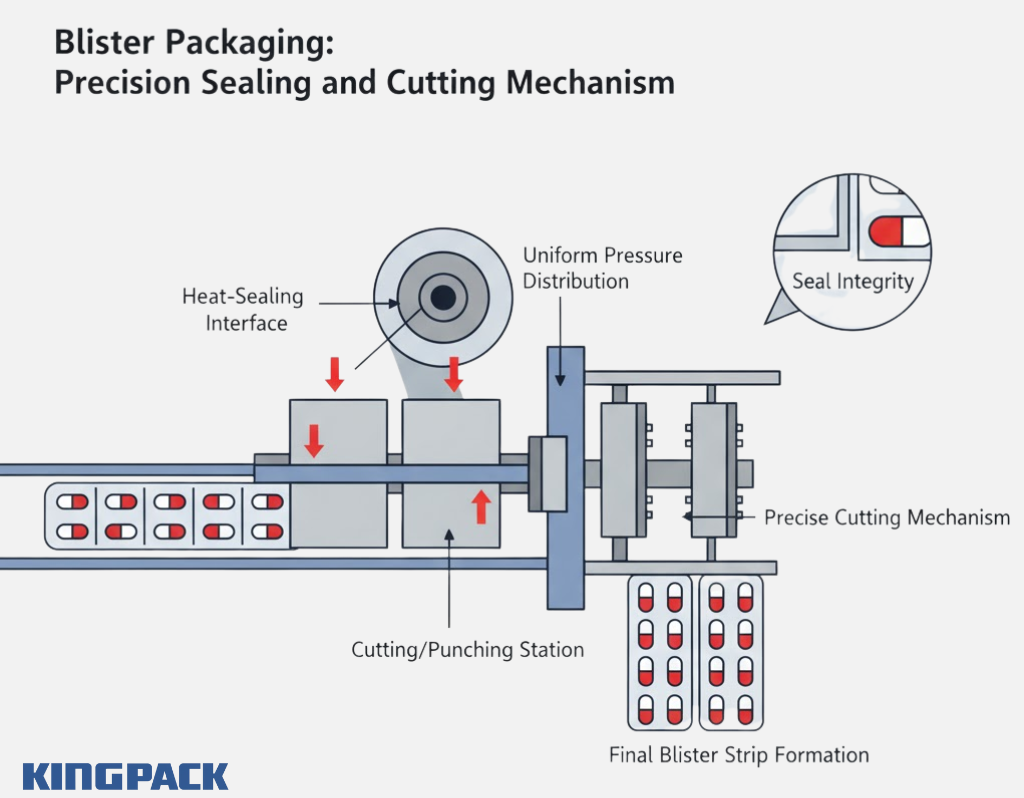
King Pack’s blister sealing systems use precise temperature calibration and servo-driven rollers for uniform pressure distribution. Once sealed, the blister web moves to the cutting station, where it’s trimmed into individual strips or cards.
The final cutting stage defines the presentation of the blister — whether in multi-unit cards or small strips. Accurate cutting prevents foil tears and maintains clean edges, which improves both appearance and usability.
Sealing and cutting highlights:
- Heat-sealing with aluminum foil or laminated film
- Cold-sealing options for temperature-sensitive products
- Servo-controlled cutters for precise trimming
- Optional perforation or embossing for easy tablet push-through
Recommended Reading: Vial Filling Machines & Processing Solutions – King Pack Aseptic Systems – King Pack Machinery
Benefits of Blister Packaging for Pharmaceuticals
Blister packs deliver strong advantages that align with pharmaceutical manufacturing’s demands:
Core benefits include:
- Enhanced shelf life and drug integrity: Blisters isolate each dose from ambient stress. Studies show that blister packaging can significantly extend stability versus bulk packaging.
- Unit‑dose administration, patient compliance & safety: Individual cavities support clear dose tracking, reduce risk of cross‑dose contamination and support tamper‑evident formats.
- Traceability, tamper evidence & visual inspection: Transparent cavities let inspectors or patients see each dose; sealed layers provide tamper resistance and make serialization easier.
These benefits make blister packaging ideal for high‑volume solid dosage forms, mission‑critical drugs, and markets with strong regulatory regimes. Choosing the right machine and material is key to realizing these advantages.
Recommended Reading: Syrup Manufacturing Full Guide – King Pack Filling & Processing Systems – King Pack Machinery
Applications of Blister Packs across Industries
Solid Oral Dosage Forms – Tablets & Capsules
Pharmaceutical tablets and capsules represent the largest application for blister packaging. Oral medications account for approximately 70% of all pharmaceutical products, and blister formats serve a majority of this segment. From antibiotics and cardiovascular drugs to pain relievers and hormonal treatments, blister packs protect diverse therapeutic categories.
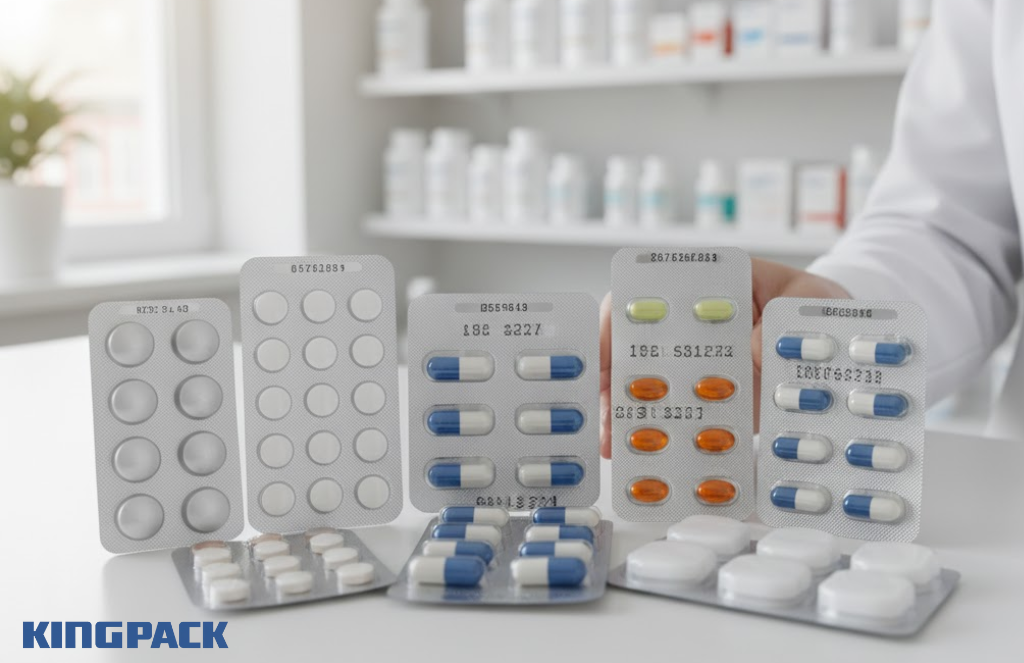
Prescription medications benefit from blister packaging’s protection and compliance features. Extended-release formulations, combination therapies, and dose-pack formats all leverage blister technology. The format also accommodates special requirements like light protection for photosensitive drugs or maximum moisture barriers for hygroscopic compounds.
Over-the-counter medications increasingly adopt blister packaging for consumer appeal and safety. The professional appearance enhances perceived quality. The unit-dose format improves safety by controlling access. The tamper evidence builds consumer confidence in product integrity.
Supplements & Nutraceuticals
Vitamin and supplement manufacturers have embraced blister packaging for similar reasons driving pharmaceutical adoption. Many supplements contain moisture-sensitive or oxygen-sensitive ingredients requiring protection. Probiotics, enzymes, and oil-based vitamins particularly benefit from the barrier properties blister packs provide.

The premium appearance of blister packaging also appeals to nutraceutical brands positioning themselves as high-quality alternatives to traditional supplements. The clear cavities showcase product appearance. The sealed format conveys freshness and quality. The unit-dose convenience appeals to consumers seeking travel-friendly supplement options.
Other Uses: Medical Devices, Small Hardware, Consumer Goods
Beyond pharmaceuticals, blister packaging serves diverse applications where individual item protection and presentation matter. Medical devices like syringes, wound dressings, and diagnostic strips use modified blister formats providing sterile barriers and easy access.
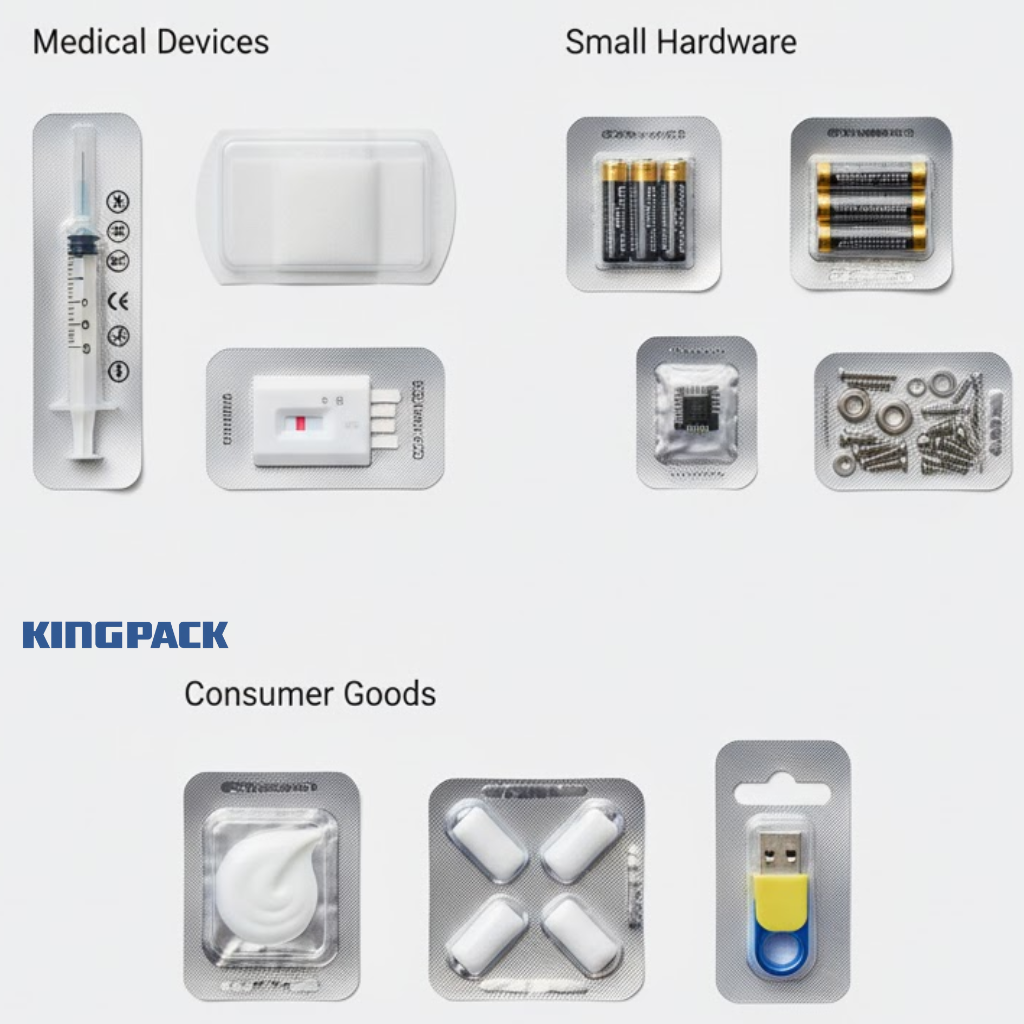
Small hardware items—screws, batteries, electronic components—often come in blister packs combining product protection with theft deterrence for retail display. The sealed format makes pilferage difficult while allowing consumers to examine products before purchase.
Consumer goods from cosmetic samples to chewing gum leverage blister packaging’s convenience and appeal. The format’s versatility extends across industries wherever individual unit protection and controlled dispensing provide value.
How to Choose the Right Blister Packaging Solution
Matching Packaging Material to Drug Sensitivity & Shelf Life Needs
Material selection begins with understanding the pharmaceutical product’s specific vulnerabilities. Stability data from development studies reveal degradation pathways—whether moisture, oxygen, light, or temperature drives product degradation. This information guides material selection to address actual protection needs without over-specifying expensive solutions.

DPP-250 Flat Blister Packaging Machine – King Pack Machinery
Moisture-sensitive formulations require materials with water vapor transmission rates appropriate to the product’s hygroscopicity and target shelf life. Standard PVC suffices for moderately stable products. PVDC-coated films suit more sensitive compounds. Cold-form aluminum handles extremely hygroscopic materials requiring maximum protection.
Oxygen-sensitive products similarly need barrier levels matching their oxidation susceptibility. Products showing minimal oxygen sensitivity may work with standard materials. Those exhibiting significant degradation require enhanced barriers from PVDC coatings or aluminum-based structures.
Cost-benefit analysis should balance material expenses against product value and required shelf life. Generic medications in competitive markets may accept shorter shelf lives with economical materials. Innovative drugs with high margins justify premium packaging extending product life and maintaining quality.
Recommended Reading: Vial Filling Machines in the Pharmaceutical – King Pack Solutions for Precision & Sterility – King Pack Machinery
Machine Selection: Speed, Format Flexibility, Change-over Capability
Production volume requirements drive machine speed specifications. High-volume products with steady demand justify investment in fast thermoforming lines producing 300-400 blisters per minute. Lower-volume or diverse product portfolios may benefit more from flexible flatbed machines offering easier changeovers despite lower speeds.
Format flexibility matters when product portfolios include multiple package sizes, cavity configurations, or frequent new product introductions. Machines with adjustable tooling and quick-change capabilities accommodate diverse requirements on shared equipment. This flexibility reduces capital investment while maintaining production efficiency.
Changeover time directly affects productivity when running multiple products. Modern blister machines with servo-driven adjustments and recipe management systems can switch formats in 15-30 minutes. Older designs requiring manual adjustments may need 2-4 hours for the same changeover. This difference substantially impacts overall equipment effectiveness when running diverse product mixes.
Supplier Credentials: Compliance (GMP, CE), After-Sales Service, Customisation
Equipment supplier selection impacts project success as much as machine specifications. Established manufacturers bring expertise solving challenges across numerous pharmaceutical applications. Their experience translates into reliable equipment designs, comprehensive documentation, and effective technical support.
Regulatory compliance credentials matter critically in pharmaceutical manufacturing. Suppliers should provide equipment designed to GMP standards with appropriate documentation packages. CE marking demonstrates European safety compliance. Comprehensive validation support—DQ, IQ, OQ documentation—facilitates regulatory approval of packaging operations.
After-sales service determines long-term success with capital equipment. Evaluate suppliers based on technical support availability, spare parts inventory, and response times for troubleshooting. Remote diagnostic capabilities enable faster problem resolution. Training programs for operators and maintenance personnel build internal expertise supporting daily operations.
Customization capability addresses unique product requirements that standard equipment configurations don’t accommodate. In-house engineering resources allow suppliers to modify machines for special cavity sizes, unusual products, or integration with existing production lines. This flexibility proves valuable as product portfolios evolve.
King Pack offers comprehensive blister packaging solutions spanning equipment selection through installation, validation, and ongoing support. Their pharmaceutical packaging expertise helps manufacturers optimize material selection, machine configuration, and line integration for diverse solid dosage products.
Conclusion
Blister packaging is a foundational technology in pharmaceutical manufacturing — combining dose protection, compliance and consumer‑friendly formats. To get it right you need the correct material, the appropriate forming/filling machine and a trusted partner. For a customised blister packaging machine and line solution — Contact King Pack today and build a production line tailored for your tablets and capsules.

