Pharmaceutical tube filling operates under some of the strictest rules in manufacturing. Every fill must meet defined limits for accuracy, cleanliness, and traceability. Equipment that falls short can trigger audit findings, production delays, or product rejection.
In this article, the focus is on how modern tube filling machines meet cGMP expectations in 2026. It explains aseptic filling requirements, cleanroom design, and the validation steps needed for global pharmaceutical approval.
The Foundations of Trust: Adhering to Global cGMP and FDA Standards
Current Good Manufacturing Practice (cGMP) regulations establish the baseline requirements for pharmaceutical manufacturing equipment. These standards exist to protect patient safety by preventing contamination, mix-ups, and errors that could compromise drug efficacy or introduce harmful substances into medications.
Non-Negotiable Machine Requirements for Cleanroom Environments
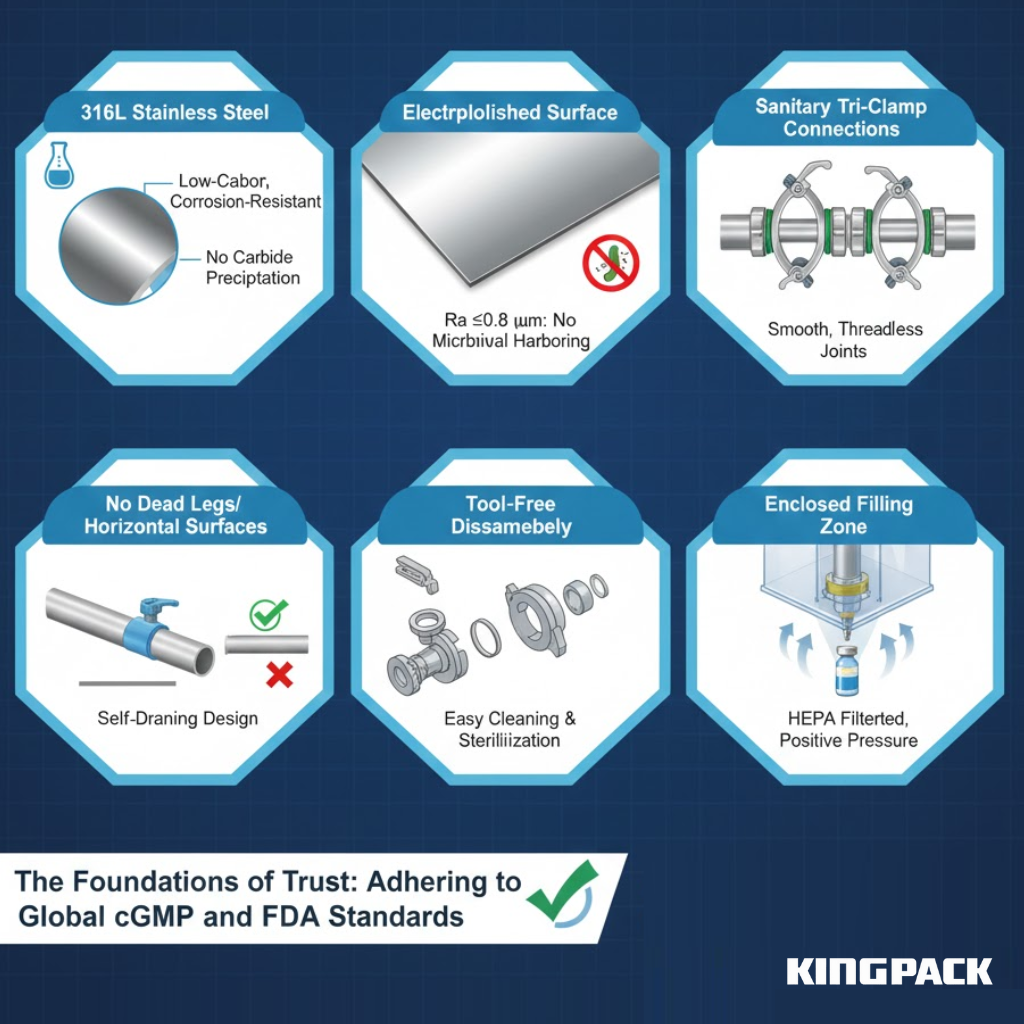
Pharmaceutical tube filling machines require design features that support cleaning, prevent contamination, and maintain complete documentation. Regulatory agencies worldwide enforce these standards through facility inspections and equipment audits.
- 316L Stainless Steel Construction: All product-contact surfaces on advanced pharmaceutical filling machines, RT60 Tube Fillers, and Aluminum tube filling and crimping machines use 316L stainless steel for superior corrosion resistance and durability.
- Electropolished Surface Finish: Surfaces achieve Ra ≤0.8 micrometers, minimizing crevices where bacteria could accumulate and simplifying cleaning validation for plastic tubes, laminate tubes, and Unit Dose squeeze tubes.
- Sanitary Connections: Tri-clamp fittings with food-grade gaskets replace threaded joints to ensure complete cleaning and prevent residue buildup.
- No Dead Legs or Horizontal Surfaces: All surfaces, including tube holders and closure stations, slope toward drain points to avoid product stagnation and microbial growth.
- Tool-Free Disassembly: Critical components such as filling nozzles, crimping machines, laminate crimpers, and closure stations can be removed without tools for cleaning and sterilization.
- Enclosed Filling Zone: HEPA-filtered enclosures maintain positive air pressure at automated filling stations, ensuring aseptic accuracy during Medium Speed or High Speed operations.
- Automated Data Logging: Control units and PLC touch screens record essential process parameters, including fill volume, seal temperature, date/lot code, expiry date, and operator ID, creating full process transparency and comprehensive batch records.
Machines meeting FDA standards typically comply with EU GMP Annex 1 and other global pharmaceutical regulations, with documentation formats adapted as needed.
Recommended Reading: What Is a Blister Pack? – Pharmaceutical Packaging Guide by King Pack – King Pack Machinery
Aseptic & Sterile Filling: Achieving Zero Contamination
Sterile pharmaceutical products require filling processes that maintain absolute microbial control throughout production. Even a single contaminated tube could cause serious patient harm, making contamination prevention the paramount concern.
King Pack’s pharmaceutical tube filling equipment incorporates aseptic filling capabilities with HEPA-filtered cleanroom environments and sterilization procedures for tubes before filling. The equipment supports CIP/SIP validation protocols required for pharmaceutical production, maintaining sterility throughout the filling process.
Technology Deep Dive: CIP (Clean-in-Place) and SIP (Sterilization-in-Place) Automation
CIP and SIP systems automate cleaning and sterilization procedures, providing superior consistency compared to manual processes while generating complete documentation for regulatory compliance.
Clean-in-Place (CIP) Systems
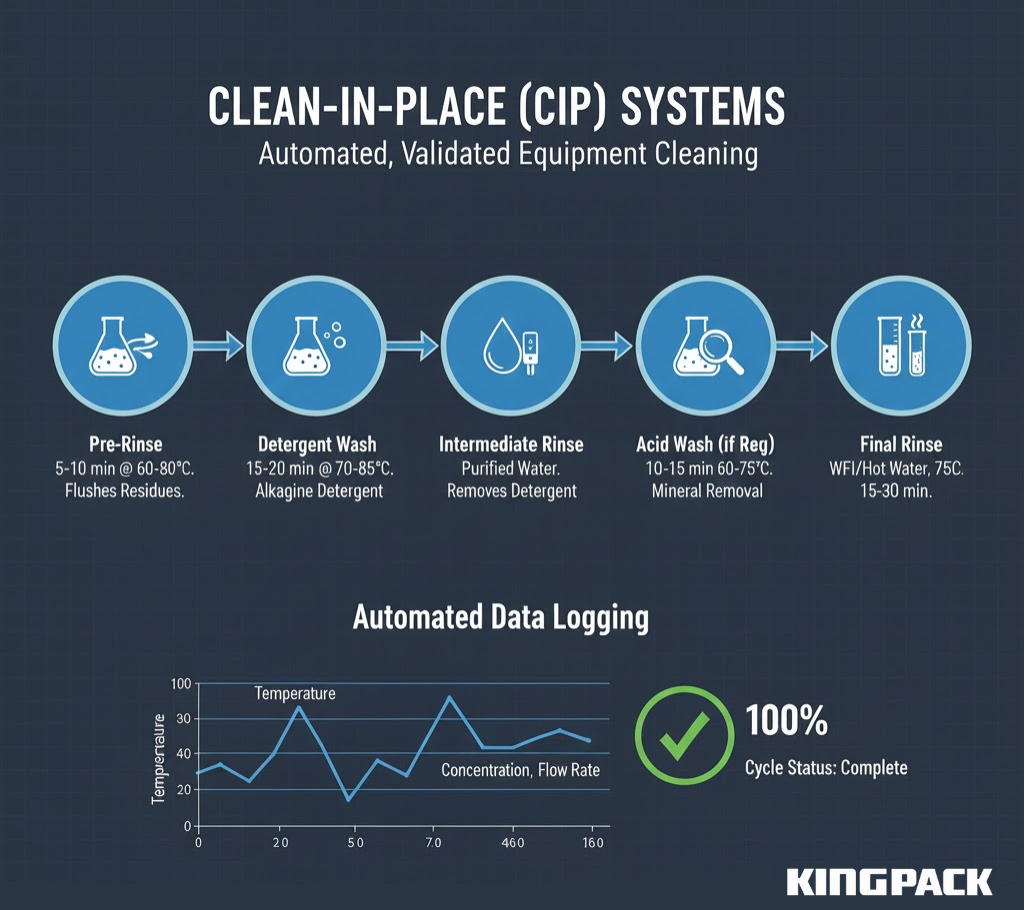
CIP systems circulate cleaning solutions through equipment without disassembly, using programmed sequences that deliver consistent results. A typical pharmaceutical CIP cycle includes:
- Pre-Rinse: Purified water flushes product residues from all surfaces (5-10 minutes at 60-80°C)
- Detergent Wash: Alkaline detergent (1-2% concentration) circulates through the system, removing organic residues and oils (15-20 minutes at 70-85°C)
- Intermediate Rinse: Purified water removes detergent residues until conductivity drops below specified limits
- Acid Wash (if required): Phosphoric or citric acid (0.5-1% concentration) removes mineral deposits and neutralizes alkaline residues (10-15 minutes at 60-75°C)
- Final Rinse: Water for Injection (WFI) removes all chemical residues until rinse water meets pharmaceutical water standards
- Sanitization: Hot water (80-85°C) or chemical sanitizer circulates through the system (15-30 minutes)
Automated systems monitor and record temperatures, concentrations, flow rates, and contact times throughout the cycle. Deviations from programmed parameters trigger alarms and prevent cycle completion until corrective actions are taken.
Sterilization-in-Place (SIP) Systems
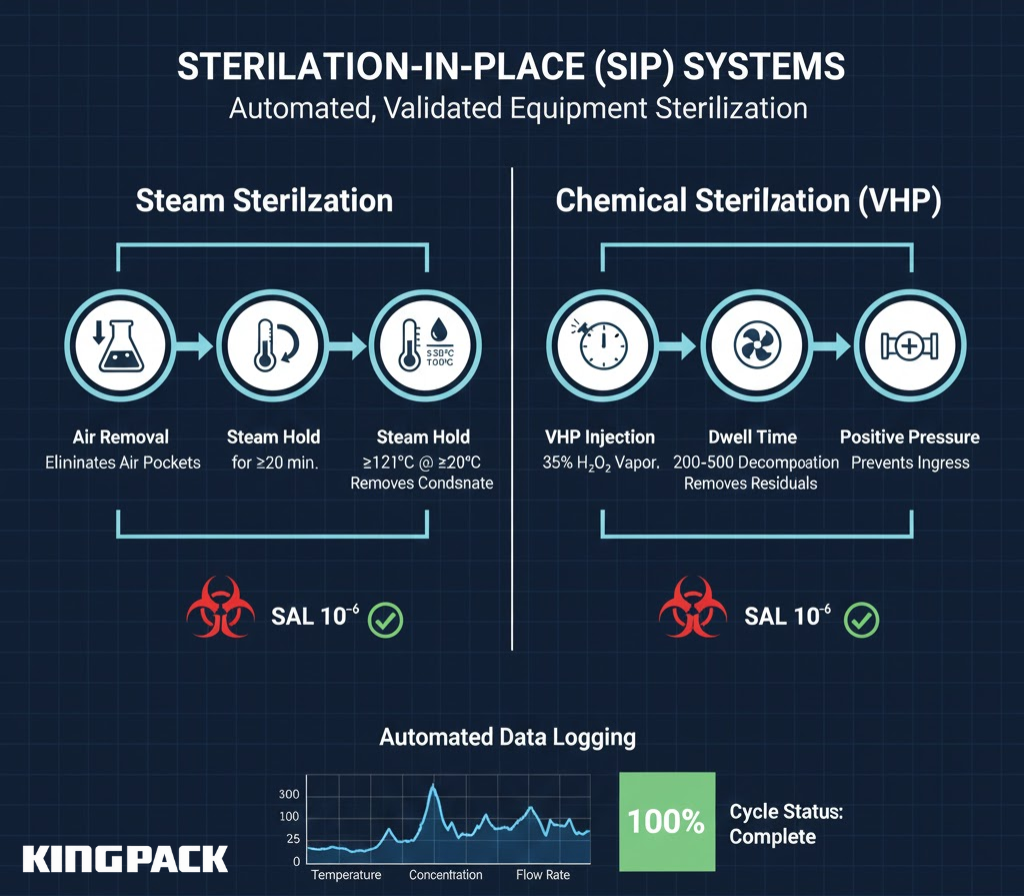
SIP systems use superheated steam or chemical sterilants to achieve sterility assurance levels (SAL) of 10⁻⁶ or better—meaning less than one chance in a million of a viable microorganism surviving sterilization.
Steam sterilization requires holding all surfaces at ≥121°C for ≥20 minutes. The system must:
- Remove all air pockets that would insulate surfaces from steam contact
- Maintain positive pressure to prevent contamination ingress during cooling
- Use condensate traps that remove water without allowing air entry
- Include temperature sensors at the coldest points to verify effective sterilization
Chemical sterilization using vaporized hydrogen peroxide (VHP) offers an alternative for temperature-sensitive components. VHP systems inject 35% hydrogen peroxide vapor into sealed chambers, maintaining concentrations of 200-500 ppm for 30-60 minutes before catalytic decomposition removes residual peroxide.
Recommended Reading: World Top 10 Vial Filling Machine Manufacturers – King Pack Global Pharma Solutions – King Pack Machinery
Micro-Dosing Accuracy: Essential for High-Value APIs and Potent Ointments
Pharmaceutical applications demand filling accuracy that exceeds standards in cosmetic or food industries. Active pharmaceutical ingredients (APIs) often cost thousands of dollars per kilogram, making overfilling economically wasteful. More critically, underfilling could deliver sub-therapeutic doses while overfilling might cause adverse effects from excessive dosing.
Pharmaceutical Dosing Standards
Regulatory agencies specify maximum acceptable dosing variation based on dose uniformity requirements:
| Product Type | Required Accuracy | Typical Technology | Validation Requirement |
| High-Potency Ointments | ±0.1% to ±0.2% | Servo piston pumps | 100% weight verification |
| Standard Ointments | ±0.5% | Piston or gear pumps | Statistical process control |
| Gels and Creams | ±1.0% | Volumetric fillers | Periodic weight checks |
High-potency compounds require the tightest tolerances. For a 10g tube of ointment containing 0.1% active ingredient (10mg total), ±0.2% accuracy means final weight must fall between 9.98g and 10.02g. This 40mg window leaves no room for equipment drift or operator error.
Technologies for Ultra-Precise Dosing
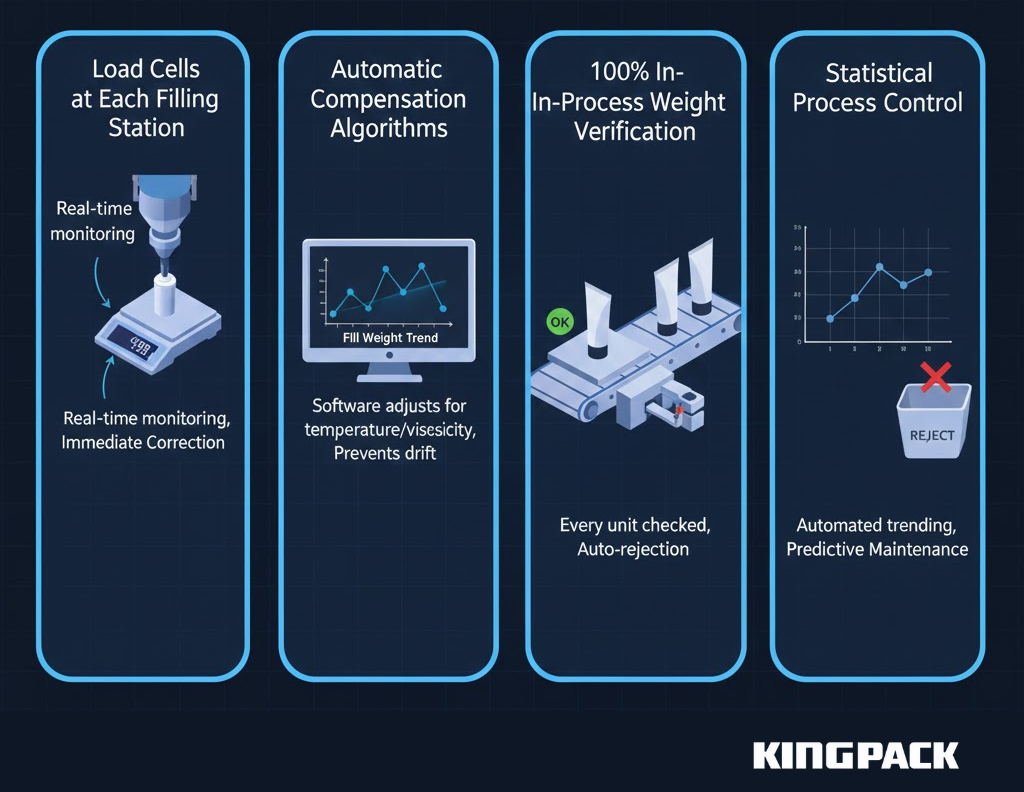
Servo-driven positive displacement pumps deliver the repeatability required for pharmaceutical applications. These systems include:
- Load Cells at Each Filling Station: Real-time weight monitoring during filling allows immediate correction if weights drift outside specifications
- Automatic Compensation Algorithms: Software adjusts fill volumes based on product temperature, viscosity changes, and observed fill weight trends
- 100% In-Process Weight Verification: Every tube passes through checkweighers immediately after filling, with automatic rejection of out-of-specification units
- Statistical Process Control: Automated trending identifies equipment drift before it produces out-of-specification product
Modern pharmaceutical tube fillers achieve dosing accuracy of ±0.1% to ±0.2% for ointments and creams, meeting the strictest regulatory requirements while minimizing product giveaway.
Recommended Reading: Types of Pharmaceutical Packaging – Comprehensive Guide by King Pack Packaging Solutions – King Pack Machinery
Validation Protocols: Your Blueprint for Machine Qualification
Equipment validation proves that machinery consistently performs as intended, producing products that meet predetermined specifications. Pharmaceutical regulations require documented validation before commercial production begins and periodic revalidation throughout equipment life.
Structured Definition: The Roles of Installation Qualification (IQ) and Operational Qualification (OQ)
Equipment validation follows a structured progression through three phases: IQ, OQ, and PQ. Each phase builds on previous work, creating comprehensive documentation that demonstrates equipment capabilities.
Installation Qualification (IQ)
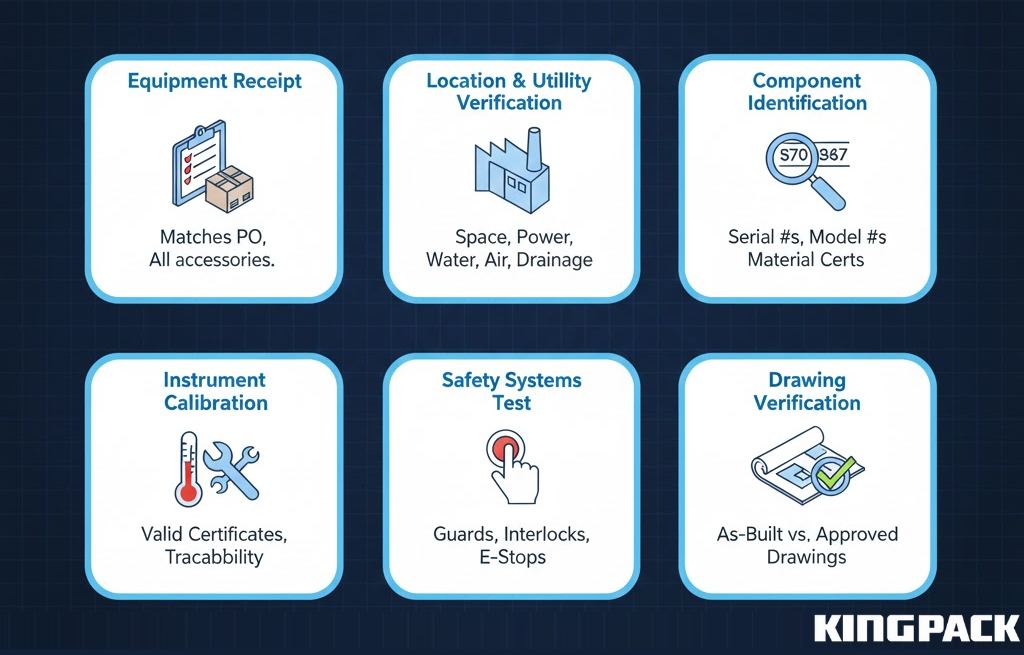
IQ verifies that equipment arrives complete and installs correctly according to specifications.
This phase documents:
- Equipment Receipt: Verification that delivered equipment matches purchase orders, including all specified options and accessories
- Location Verification: Confirmation that installation site provides adequate space, utilities, and environmental controls
- Utility Connections: Documentation of electrical connections, compressed air supply, water supplies, and drainage systems
- Component Identification: Recording serial numbers, model numbers, and material certificates for all equipment components
- Instrument Calibration: Verification that all measuring instruments (temperature sensors, pressure gauges, load cells) arrive with valid calibration certificates traceable to national standards
- Safety Systems: Testing of all guards, interlocks, and emergency stop functions
- Drawing Verification: Confirmation that as-built equipment matches approved engineering drawings
IQ requires substantial documentation but minimal actual testing. The focus is verification rather than performance evaluation. A typical IQ protocol contains 50-100 individual test points, each requiring objective evidence (photographs, certificates, instrument readings) supporting the verification.
Recommended Reading: Knowledge of Pharmaceutical Blister Packaging Machines – King Pack Packaging Solutions – King Pack Machinery
Operational Qualification (OQ)
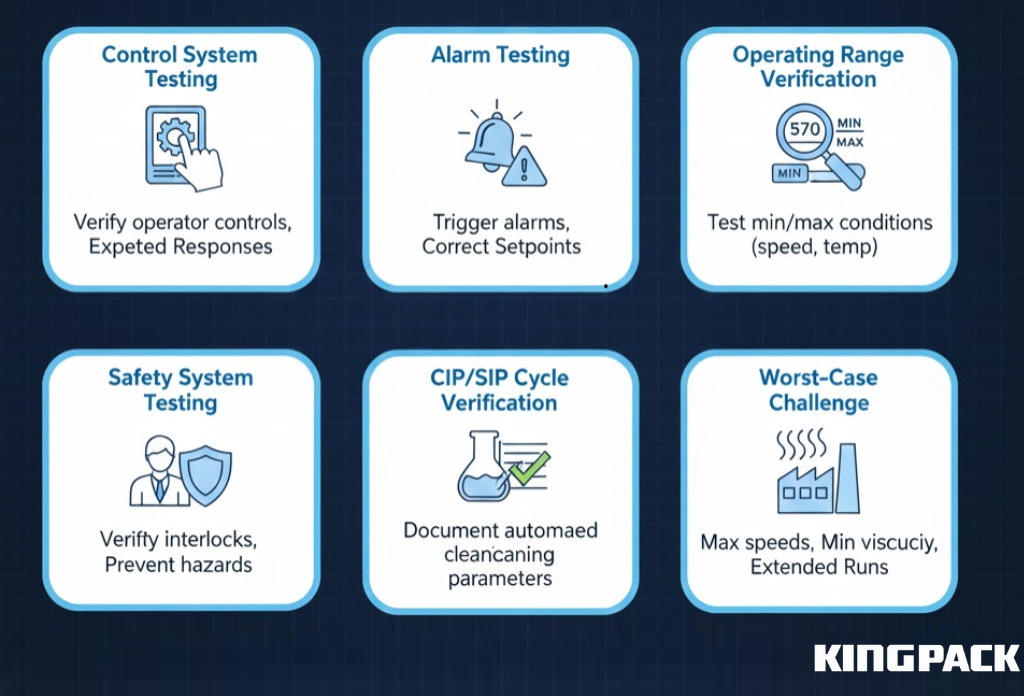
OQ verifies that equipment performs correctly across its intended operating ranges. This phase tests all functions and ensures control systems respond properly to different inputs:
- Control System Testing: Confirms that PLC touch screens, control panels, and automated filling functions operate as designed, producing expected equipment responses.
- Alarm Testing: Activates all alarms on Tube filling and sealing machines, RT60 Tube Fillers, and sealing machines to verify they trigger at setpoints and alert operators appropriately.
- Operating Range Verification: Tests equipment at minimum, nominal, and maximum speeds, pressures, and temperatures, including High Speed and Medium Speed operations.
- Safety System Testing: Ensures tube elevators, tube holders, closure stations, and other safety interlocks prevent hazardous conditions during operation.
- CIP/SIP Cycle Verification: Documents that cleaning and sterilization cycles achieve specified parameters for semi-solid products, dense fluids, plastic tubes, laminate tubes, and collapsible aluminum tubes.
- Worst-Case Challenge Testing: Operates equipment under maximum speeds, low-viscosity products, and extended run times to verify consistent performance.
OQ usually uses placebo materials (water or glycerin) rather than actual product. The goal is confirming functionality, not product quality. Each test has pre-defined acceptance criteria, with objective measurements showing pass/fail results.
Performance Qualification (PQ): Documenting Consistent, Repeatable Production
PQ proves that equipment consistently produces products meeting all quality specifications when operated by trained personnel using actual materials and procedures. This phase represents the final validation step before commercial production begins.
PQ Execution Requirements
Regulatory agencies typically require three consecutive successful production runs to demonstrate consistency:
| PQ Requirement | Acceptance Criteria | Documentation |
| Fill Weight Uniformity | 100% of tubes within ±specified tolerance | Individual tube weights for entire run |
| Seal Integrity | Zero leaks detected through validated testing | Leak test results for minimum sample size |
| Batch Documentation | Complete, accurate records for all parameters | Batch production records with all signatures |
| Environmental Monitoring | All cleanroom parameters within specifications | Particle counts, microbial samples, temperature/humidity logs |
| Equipment Performance | OEE ≥target value, no unexplained stoppages | Production logs showing speed, downtime, rejection rates |
Critical Process Parameters
PQ identifies and documents Critical Process Parameters (CPPs)—settings that significantly impact product quality. For tube filling, CPPs typically include:
- Fill volume setpoint and operating range
- Product temperature during filling
- Seal temperature and dwell time
- Line speed and indexing timing
- Nitrogen flush concentration (if used)
Equipment must maintain all CPPs within validated ranges throughout commercial production. Changes to CPPs require revalidation to verify that modified parameters still produce acceptable product.
Continued Process Verification (CPV)
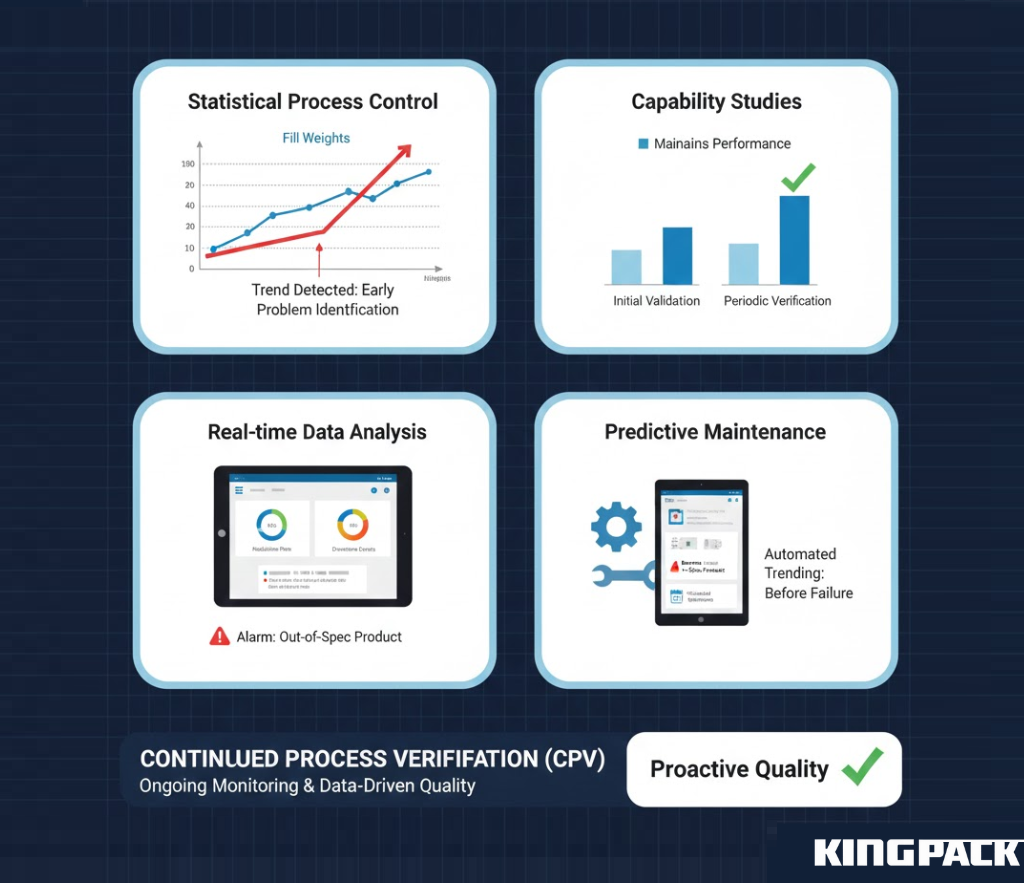
Modern GMP guidelines emphasize ongoing verification throughout the product lifecycle rather than one-time validation at equipment installation. CPV programs collect and analyze production data continuously, identifying trends that might indicate developing problems before they produce out-of-specification product.
Statistical process control charts track fill weights, rejection rates, downtime events, and other metrics. Periodic capability studies verify that equipment maintains validated performance levels. This data-driven approach provides earlier problem detection than periodic revalidation schedules.
Conclusion: Investing in Quality and Compliance for Your Pharma Future
Tube filling machines for pharmaceutical use must meet strict standards from day one. Materials, surface finish, airflow control, and data logging all play a role in compliance. Equipment adapted from other industries struggles under regulatory review. Pharma-grade design simplifies inspections and daily operations.
Validation completes the picture. Structured protocols confirm repeatable performance under real production conditions. CIP, SIP, and micro-dosing systems work together to maintain control at every step. This approach supports consistent output and protects both patients and brands.
Recommended Reading: Vial Filling Machines in the Pharmaceutical – King Pack Solutions for Precision & Sterility – King Pack Machinery
Request a Validated Consultation on Pharmaceutical Filling Equipment
King Pack offers technical consultation services backed by extensive experience in pharmaceutical tube filling applications. Our team can assess your specific requirements and recommend equipment configurations that balance compliance, performance, and cost considerations. Contact our sales team to schedule a consultation and discuss your pharmaceutical packaging needs!
Frequently Asked Questions (Pharma FAQs)
What is the difference between Aseptic and Sterile filling in the pharmaceutical context?
Aseptic filling sterilizes the product, container, and closure separately, then fills them in a controlled clean environment. Sterile filling uses terminal sterilization after sealing the filled container. Aseptic filling suits heat-sensitive products, while terminal sterilization fits heat-stable ones.
Why is Stainless Steel 316L mandatory for pharmaceutical contact parts?
316L stainless steel resists corrosion from pharma products and cleaning chemicals. Its low carbon content prevents weld-related corrosion. The material tolerates repeated CIP and SIP cycles without contaminating the product.
What are the three main stages of the machine validation process (IQ, OQ, PQ)?
IQ confirms correct installation and documentation. OQ verifies machine functions across operating ranges. PQ proves consistent production quality under real manufacturing conditions using trained operators.
How do tube fillers prevent cross-contamination between different drug batches?
Validated CIP cycles remove product residues between runs. Enclosed filling zones and clean design reduce contamination risk. Batch tracking and cleaning records prevent mix-ups and support audits.

