Pharmaceutical packaging protects medications from environmental factors while providing clear product identification and tamper evidence.
Among various packaging formats, blister packs have become the industry standard for solid oral dosage forms. These machines transform flat film into individual dose protection, combining efficiency with patient safety.
Understanding blister packaging technology helps pharmaceutical manufacturers choose appropriate equipment for their production needs. This guide covers machine types, working principles, materials, and selection criteria for pharmaceutical blister packaging systems.
What Is a Blister Packaging Machine?
Definition and Purpose of Blister Packaging
A blister packaging machine forms cavities in plastic film, fills them with pharmaceutical products like tablets or capsules, and seals them with backing material. Each cavity, or “blister,” protects individual doses from moisture, oxygen, light, and contamination.
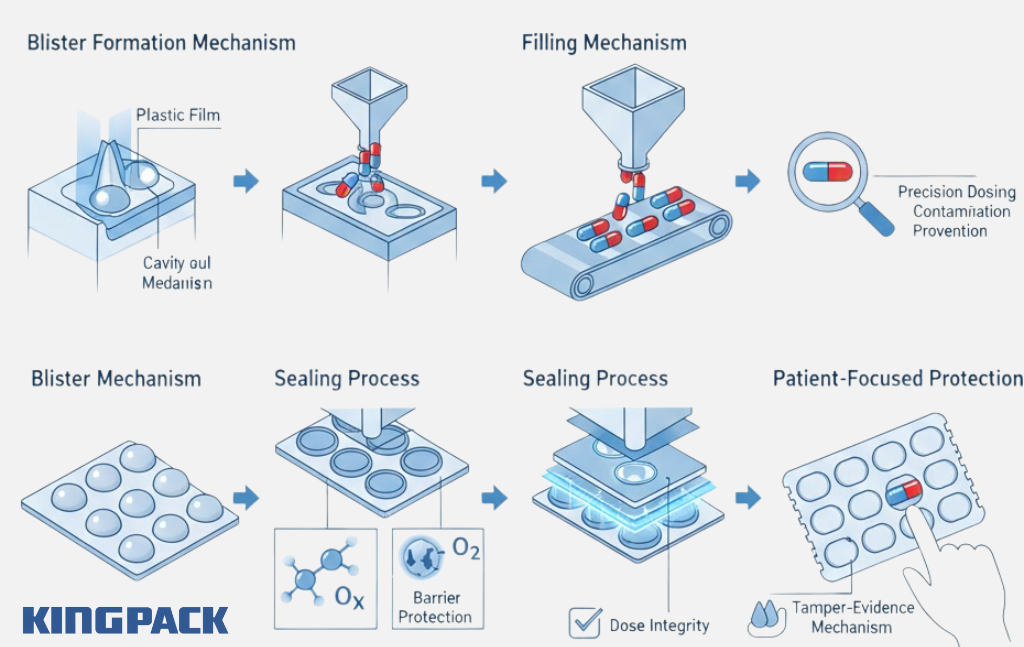
The primary purpose extends beyond protection. Blister packs enable accurate dose counting, prevent cross-contamination between units, provide tamper evidence, and allow visual inspection without opening the package. For patients, blister packs simplify medication adherence by clearly showing which doses remain.
Historical Development and Usage in Pharmaceuticals
Blister packaging emerged in the 1960s as pharmaceutical companies sought better protection than bulk bottles. Early machines were simple, manually operated devices that formed and filled one blister at a time.
Research from recent studies shows that the primary role of pharmaceutical blister packaging is protecting the drug product, especially from moisture. Studies found that blister packs built with high-barrier materials (such as PVC/Aclar® or PVC/PVdC) significantly slow down moisture ingress and resulting degradation of tablets. While modern production doesn’t rely solely on one packaging form, the trend toward automated blister packaging reflects its key role in preserving drug quality and shelf‑life.
Types of Blister Packaging Machines
Roller Blister Packaging Machine
Roller-type machines represent the most common blister packaging configuration in high-volume pharmaceutical production. These systems use continuous motion, with film unwinding from rolls and passing through forming, filling, sealing, and cutting stations in one continuous process.
DPH-260 High-Speed Blister Packaging Machine – King Pack Machinery
The roller design allows for high-speed operation, typically processing 200-400 blisters per minute depending on configuration. Film moves horizontally through synchronized stations, with each operation timed precisely to maintain continuous flow.
Key characteristics of roller machines:
- Continuous motion operation for high throughput
- Suitable for large-volume production runs
- Lower unit cost per blister at high volumes
- Requires longer setup time for format changes
- Best for standardized products with consistent demand
Flatbed Blister Packaging Machine
Flatbed blister machines are ideal for medium-volume production or products that require flexible formats. Unlike roller machines, the forming film moves intermittently through the stations, stopping at each stage for precise filling and sealing. This makes them suitable for smaller batches, specialty products, or tablets and capsules with irregular shapes.

DPP-250 Flat Blister Packaging Machine – King Pack Machinery
Flatbed machines are also easier to adjust for different formats, allowing faster changeovers and reducing downtime when switching products. While their speed is lower than roller machines, they provide high accuracy and control, making them a preferred choice for customizable or multi-spec production lines.
Key characteristics of flatbed machines:
- Intermittent motion for precise forming, filling, and sealing
- Suitable for medium-volume or specialty products
- Faster format changeovers and multi-spec capability
- High fill accuracy for irregular shapes or sensitive tablets
- Ideal for lines that require frequent product or packaging changes
Roller-Plate Blister Packaging Machine (Hybrid Type)
Hybrid blister machines combine the speed of roller systems with the flexibility of flatbed machines. The forming film typically moves continuously through the forming station but stops intermittently for filling or sealing. This allows the line to handle both standard high-volume products and specialized formats with minimal compromise on speed or quality.
These machines are increasingly popular in modern pharmaceutical facilities that require a single line to accommodate multiple product types. Hybrid designs optimize throughput while maintaining precise filling, sealing, and batch coding, making them a versatile choice for multi-product lines.
Key characteristics of hybrid machines:
- Combines continuous forming with intermittent filling/sealing
- Supports both high-volume and specialty products
- Offers faster format changeover than pure roller systems
- Maintains high fill and seal precision
- Suitable for flexible production lines with variable demand
How Does Blister Packaging Work Step-by-Step?
Step1: Forming of Blister from Film
The process begins with plastic film unwinding from a roll and entering the forming station. Here, heat softens the film while vacuum or compressed air pulls it into mold cavities, creating the blister pockets that will hold pharmaceutical products.
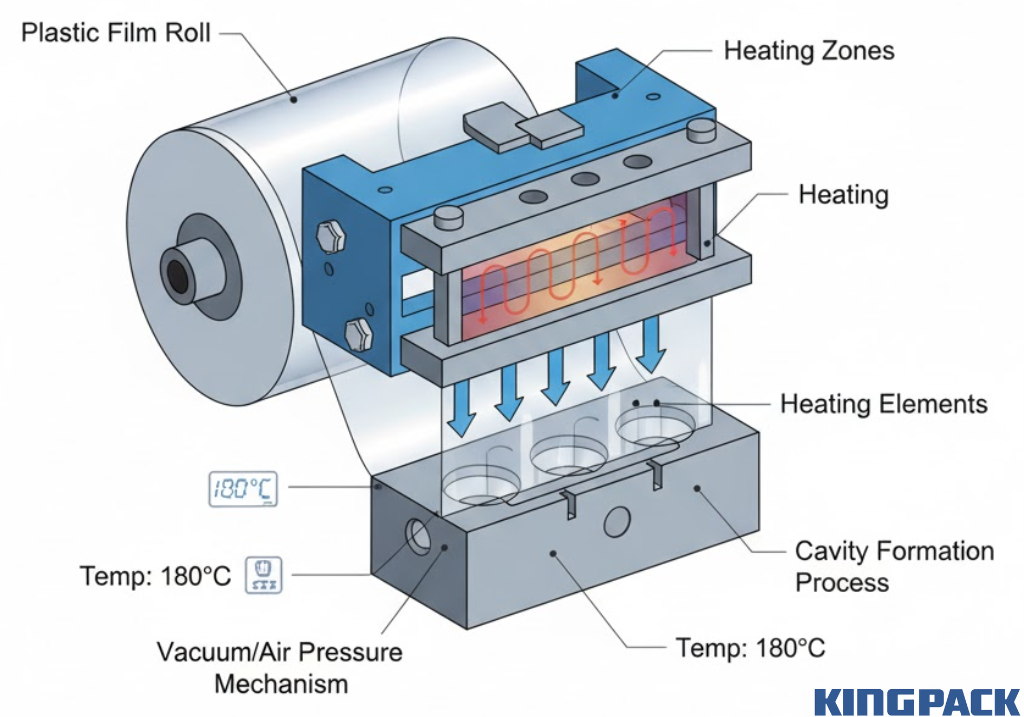
Temperature control is critical during forming. Too little heat leaves film rigid and prone to cracking. Excessive heat degrades the material or creates thin spots that compromise barrier properties. Modern machines use precise temperature zones and programmable heating profiles for different film types.
Step 2: Filling Tablets/Capsules into Blister Cavities
Once formed, blisters move to the filling station where tablets or capsules drop into each cavity. Filling mechanisms vary by machine type and product characteristics. Common methods include gravity feed from hoppers, pick-and-place systems using vacuum or mechanical fingers, and vibratory feeders for small products.
Fill accuracy matters both for regulatory compliance and patient safety. Vision systems integrated into modern blister machines verify that each cavity contains the correct product before sealing. Missing or damaged products trigger rejection systems that remove defective blisters from the production line.
Step 3: Sealing & Printing/Batch-Coding
After filling, backing material (typically aluminum foil or paper-foil laminate) feeds over the filled blisters. Heat and pressure seal the backing to the formed plastic, creating individual enclosed pockets for each dose.
Sealing parameters require careful optimization. Insufficient heat or pressure creates weak seals that fail during handling. Excessive parameters can burn the backing material or melt the plastic film, compromising package integrity.
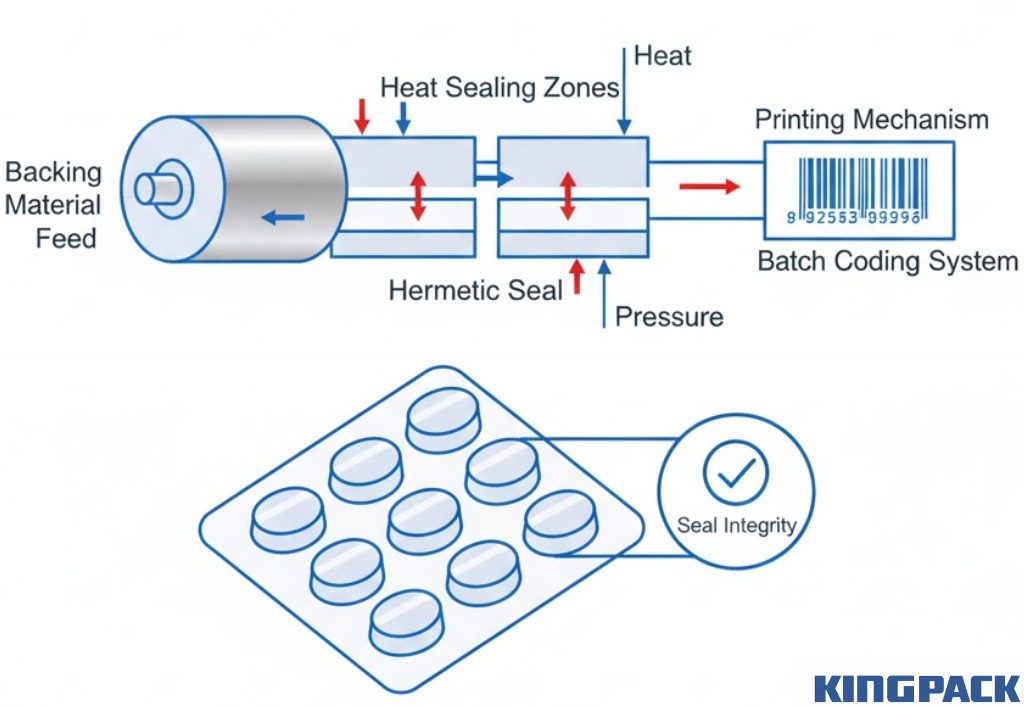
Printing and batch coding typically occur during or immediately after sealing. Information like batch numbers, expiry dates, product names, and dosage strength prints directly on the backing material. Some machines also apply graphics or brand elements during this stage.
Step 4: Punching or Die-Cutting into Blister Sheets or Units
The final step separates individual blisters or blister cards from the continuous web. Die-cutting stations use shaped punches to cut specific configurations, whether individual blisters, strips of 10 units, or cards containing multiple blisters arranged in calendar-pack formats.
Waste material from the punching process gets wound onto take-up rolls for disposal or recycling. Quality systems monitor this waste stream, as excessive scrap often indicates problems with forming, filling, or sealing operations upstream.
Blister vs Strip Packaging
Strip packaging offers advantages in material efficiency and package size but typically provides lower barrier protection than rigid blister cavities. The following comparison highlights key differences:
| Feature | Blister Packaging | Strip Packaging |
| Material Usage | Higher (rigid cavity + backing) | Lower (two flexible layers) |
| Moisture Barrier | Excellent (with proper film selection) | Good to moderate |
| Product Visibility | Clear through transparent cavity | Limited or none |
| Package Rigidity | High, protects fragile products | Low, products require structural strength |
| Dispensing | Individual units or multi-unit cards | Individual units (tear-off) |
| Cost per Unit | Higher at low volumes | Lower across all volumes |
| Shelf Life | Extended (18-36 months typical) | Moderate (12-24 months typical) |
When to Choose Blister vs Strip for Pharmaceuticals
Blister packaging suits products requiring maximum protection, especially moisture-sensitive medications like effervescent tablets or hygroscopic APIs. Products marketed in developed countries with established cold chains and distribution systems benefit from blister packs’ visibility and consumer appeal.
Strip packaging works well for cost-sensitive markets, hospital unit-dose distribution, and products with inherent stability. Generic medications in price-competitive markets often use strip packs to reduce packaging costs while maintaining acceptable protection.
Recommended Reading: Everything You Need to Know About Vial Packaging Machines – King Pack Machinery
Materials Used in Blister Packaging
Common Materials: PVC, PVDC, Composite Films
PVC (Polyvinyl Chloride) serves as the standard forming film for pharmaceutical blisters. It offers good formability, clarity, and cost-effectiveness. Standard PVC provides basic moisture and oxygen barriers suitable for many stable drug formulations.
PVDC (Polyvinylidene Chloride) coated PVC significantly improves barrier properties. The PVDC coating adds a high-barrier layer that dramatically reduces moisture and oxygen transmission. This enhanced protection extends shelf life for sensitive medications but increases material cost.
Composite films combine multiple materials to achieve specific barrier properties. Aclar (PCTFE) laminates provide exceptional moisture protection for highly hygroscopic drugs. Aluminum-based cold-form materials offer absolute light, moisture, and oxygen barriers but require specialized forming equipment.
Backing materials typically consist of aluminum foil, which provides excellent barrier properties, or paper-aluminum laminates that combine printability with protection. Thickness typically ranges from 20 to 45 microns, depending on required barrier levels.
Recommended Reading: How Vial Filling Machines Improve Efficiency and Accuracy in Liquid Filling – King Pack Machinery
Barrier Properties: Moisture, Oxygen, Light, Chemical Resistance
Different pharmaceutical products require specific barrier characteristics based on their degradation pathways. A recent study analyzed how packaging materials affect drug stability across various environmental stresses. Moisture sensitivity drives material selection for many pharmaceuticals. Hygroscopic drugs like aspirin, ranitidine, and many antibiotics degrade rapidly when exposed to humidity.
PVDC-coated films or cold-form aluminum provide the protection these products need.
Oxygen sensitivity affects products containing unsaturated compounds or those prone to oxidation. Vitamins, antioxidants, and some cardiovascular medications require high oxygen barriers. Aluminum-based materials or composite films with metallized layers address this need.
Light sensitivity concerns photolabile drugs that degrade under UV or visible light exposure. Aluminum backing materials provide complete light blockage. For transparent blisters, amber-tinted PVC or light-blocking PVDC coatings offer protection while maintaining some product visibility.
Material Selection Criteria for Pharmaceutical Applications
Choosing the right blister material involves considering:
- Drug stability from accelerated aging studies
- Target shelf life under expected storage conditions
- Regulatory requirements in intended markets
- Cost and pricing pressures
- Sustainability and recyclability goals
- Compatibility with inline machines, in-line blister packaging, automatic blister feeders, Deblister Machines, and other feeding and handling systems
King Pack’s technical team helps customers select materials based on specific product needs and market conditions, ensuring seamless integration with monobloc blister packaging, Blister seal machines, shuttle seal machines, and roller sealing or heat-seal blisters systems. Advanced features like Sealing pallets, plate sealing, and pinhole detectors further enhance packaging quality and line efficiency, whether using push-through packs or coldform blisters.
Recommended Reading: What Is a Prefilled Syringe Filling Machine? – King Pack Machinery
Advantages of Blister Packaging Machines
Blister packaging keeps tablets, capsules, and medical devices safe by sealing each dose individually, protecting them from moisture, dust, and physical damage. King Pack’s fully automatic and semi-automatic rotaries, along with manual shuttle machines, ensure precise and consistent sealing, maintaining product integrity from production to patient use. Advanced servo technology enhances accuracy and efficiency, even for varying batch sizes or Small Batch production runs.
Key Advantages:
- Protection: Each dose remains sealed until use, preventing degradation and contamination.
- Inspection & Traceability: Thermoform blisters with clear cavities allow easy verification of dosage and damaged units, helping patients track daily intake.
- Efficiency: Fully automatic rotaries and integrated cartoning machines optimize line performance and production costs, while reducing packaging weight and shipping expenses.
- Flexibility: Machines support different batch sizes and production needs, demonstrating high line competence across varied product types.
These features make blister packaging the ideal choice for pharmaceuticals and medical devices, delivering safe, traceable, and cost-effective packaging. King Pack machines combine reliability with efficiency, ensuring high-quality output for any production scale.
How to Select a Reliable Blister Packaging Machine
Manufacturer Experience & Customisation Ability
Look for a machine supplier with proven experience in pharmaceutical packaging. Customisation options matter—especially if your line handles different formats, doses or materials. For example, King Pack offers customised solutions for filling, sealing and packaging lines.
Machine Speed, Format Changeover, Multi‑spec Capability
Key performance metrics include cycle speed (blisters per minute), ability to change format/spec quickly, and multi‑spec capability. A recent study shows that the pharma blister machine market is expected to grow from USD 2.4 billion in 2025 to USD 3.2 billion by 2035, at a CAGR of 2.8%—highlighting that machine manufacturers are under pressure to deliver high‑efficiency, flexible systems.
Compliance with GMP / FDA / CE Standards and After‑Sales Support
Machines must meet GMP, FDA, CE and ISO standards in materials, design, sealing integrity and documentation. After‑sales service, spare parts supply and training are also important. A 2025 study on blister packaging operations found that optimization of machine design significantly improved operational performance in pharmaceutical lines.
Recommended Reading: Why Tube Sealing Is Essential for Ophthalmic Ointment Production – King Pack Machinery
Conclusion
Blister packaging machines stand at the heart of efficient, safe, and compliant pharmaceutical packaging lines. Their role spans forming, filling, sealing, coding and inspection—bringing together product integrity, patient safety, and regulatory compliance.
With rising global demand, the blister packaging market is set for strong growth in the coming years. Partnering with a machine manufacturer like King Pack that offers tailored, high‑precision solutions can help your facility meet speed, flexibility and quality targets.
For customised blister packaging machine solutions, contact King Pack today and ensure your line is ready for current and future demands.

