Syrup production may sound simple — just sugar and water — but industrial-scale manufacturing demands precision, hygiene, and the right equipment at every step. From mixing to filling, every stage can affect viscosity, clarity, stability, and shelf life.
In this guide, we will walk you through the full syrup-manufacturing process, highlight critical quality control points, and show how King Pack’s filling and processing systems deliver reliable, food-grade solutions.
Essential Ingredients and Formulation Basics
Key Syrup Ingredients (Sugar, Water, Acids, Flavorings)

Syrup production depends on precise ingredient balance. While sugar and water form the foundation, the supporting ingredients — acids, preservatives, and flavorings — define texture, shelf life, and sensory appeal.
Each component affects not only sweetness and viscosity but also how the syrup behaves during processing, filling, and long-term storage. For manufacturers, ingredient choice directly influences clarity, stability, and microbial safety.
| Ingredient | Primary Role | Typical Use or Range | Processing Considerations |
| Sugar (Sucrose, Glucose, or HFCS) | Core sweetening and viscosity agent | 60–85% Brix (depends on product type) | Heating must achieve full dissolution; excess sugar can crystallize during cooling |
| Water (Purified/De-ionized) | Solvent and carrier medium | Varies by formula | Must be free of minerals and microbes to prevent haze or spoilage |
| Acids (Citric, Phosphoric, etc.) | pH control, flavor balance, microbial inhibition | pH 3–4 for most food/pharma syrups | Added after sugar dissolution to avoid inversion or unwanted reactions |
| Preservatives (Sodium Benzoate, Potassium Sorbate) | Extend shelf life by limiting microbial growth | 0.05–0.1% | Added after cooling to retain effectiveness |
| Flavorings & Colorants | Define taste, aroma, and visual appeal | Minimal concentration | Added at final mixing to avoid degradation during heating |
Even with standard recipes, formulation adjustments are essential to match end-use requirements. Beverage syrups, for example, prioritize clarity and pourability, while pharmaceutical syrups focus on stability and uniform dosing.
Flavor retention also depends on the sealing and filling conditions — exposure to high temperatures or oxygen can degrade volatile compounds. The key to consistent syrup quality lies in ingredient compatibility, process control, and the precise dosing capabilities of systems like King Pack’s automatic syrup mixing and filling lines.
Formulation Considerations (Viscosity, Flavour, Use Case)
Formulating a syrup involves more than combining sweeteners and flavoring agents. Each application demands a specific balance of texture, stability, and performance. Viscosity, for example, determines not only the consumer’s sensory experience but also how efficiently the syrup moves through pumps, filling nozzles, and packaging lines.
A thick dessert syrup requires more robust agitation and positive displacement filling systems to maintain consistency, while thinner beverage or medicinal syrups can use gravity or piston-based fillers. In both cases, viscosity control depends on temperature, sugar concentration, and ingredient ratios — factors that must remain consistent across every batch.
Flavor stability is another challenge in syrup design. Many flavor compounds are volatile and degrade when exposed to heat or oxygen during processing. Using encapsulated flavorings or adding them at the final cooling stage helps preserve aroma and taste.
In a study found that up to 40% of natural fruit flavor loss in syrups occurs when exposed to 80°C for more than five minutes. To counter this, manufacturers rely on closed-loop mixing systems with minimal oxygen exposure, such as those offered by King Pack’s vacuum syrup preparation units, which help retain sensory integrity while improving batch uniformity.
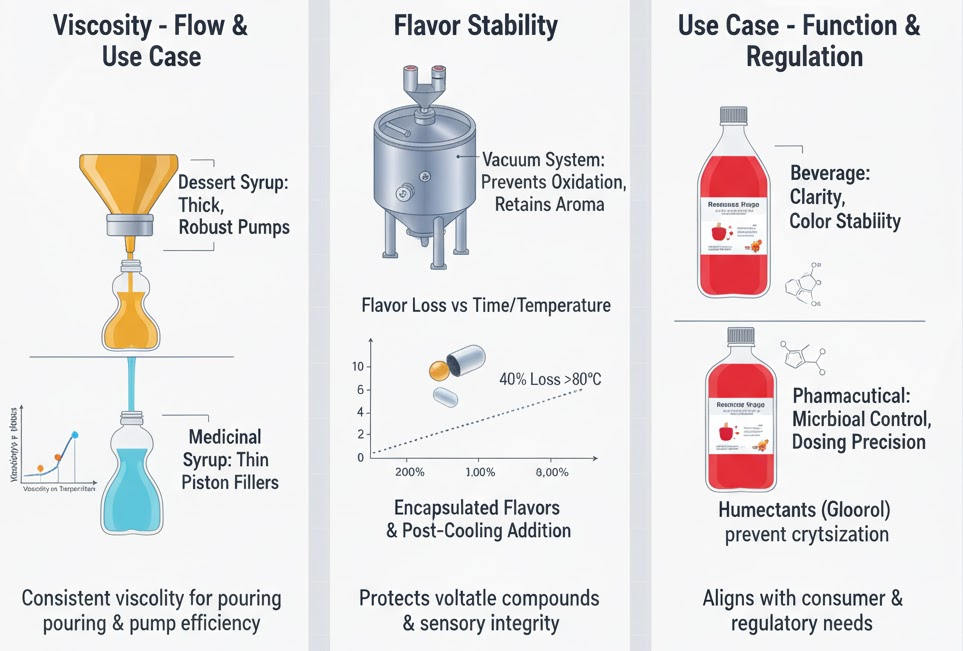
Finally, the intended use case defines the formulation’s technical and regulatory boundaries. Beverage syrups focus on clarity, color stability, and pourability, while pharmaceutical syrups require microbial control and precise dosing. For example, oral medicinal syrups often contain humectants like glycerol or sorbitol to maintain uniform viscosity and sweetness without crystallization.
On the other hand, dessert or flavoring syrups prioritize visual appeal and thermal stability during storage. Aligning formulation with use case ensures that the final product meets both consumer expectations and production efficiency standards.
Recommended Reading: How to Choose a Tube Filling Machine Brand: In-Depth Analysis of Four Major Regions – King Pack Machinery
Syrup Production Workflow
Once your ingredients are selected and formulation settled, the next major challenge is translating the recipe into a scalable, efficient workflow.
Preparation of Ingredients
Before mixing can begin, all raw materials have to be prepared. Sugar must be weighed, water must be measured and purified, and acids or flavors must be pre-diluted or preheated.
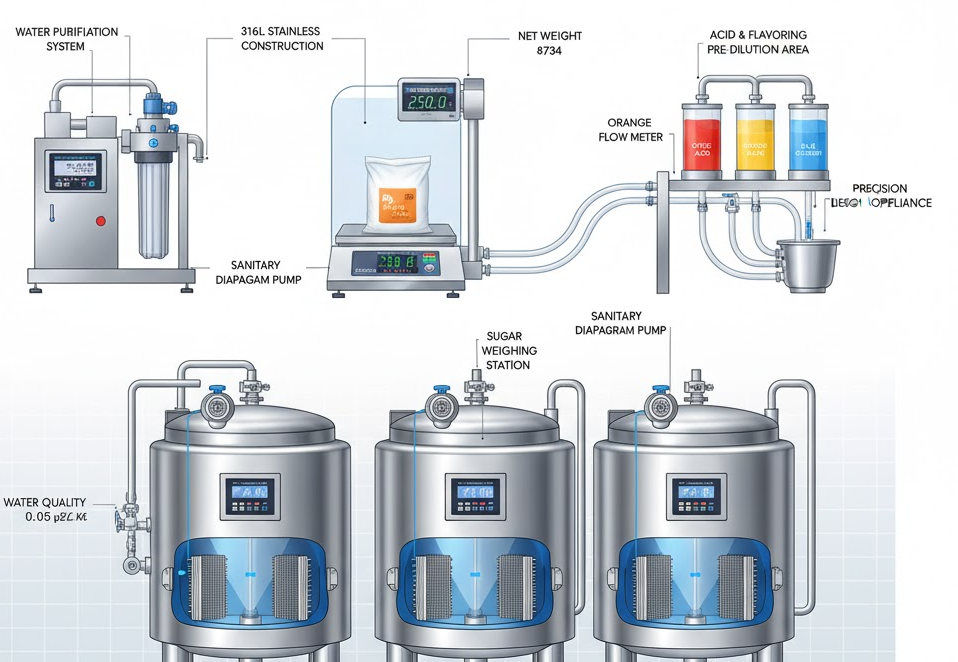
In a properly designed plant, this is done in buffer tanks or holding vessels with sanitary design.
- Many syrup plants use buffer tanks with jacketed walls, allowing pre-heating of water or partial dissolution of sugar before final blending.
- Ingredients are typically added in a controlled order: water → sugar → acids → flavoring, to minimize precipitation and ensure rapid dissolution.
- For food-grade operations, all tanks should be made from stainless steel (typically 304 or 316L), in compliance with hygiene standards, to avoid contamination or corrosion.
Mixing & Dissolving
The mixing stage is central to syrup production, where sugar and other ingredients blend with water to create a uniform, stable solution. Complete dissolution and proper agitation are key to avoiding undissolved crystals or trapped air that can affect clarity and taste.
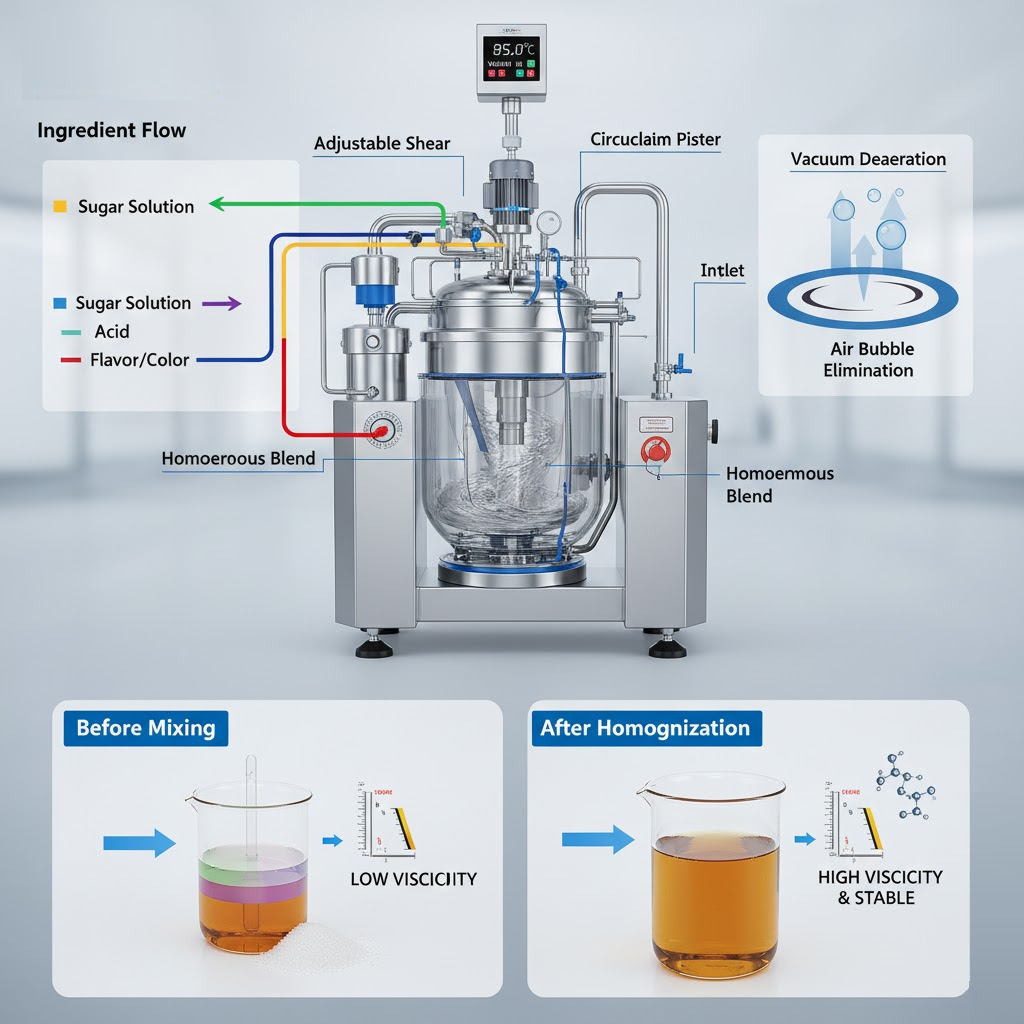
For thicker or high-Brix syrups, high-shear mixing ensures consistent blending of dense sugar solutions and additives like acids and flavorings across every batch. King Pack’s KPZ-series vacuum homogenizing emulsifiers streamline this process with adjustable shear, temperature control, and built-in vacuum technology.
These features eliminate air bubbles, prevent foaming, and improve clarity. The result is a smooth, transparent syrup with stable viscosity, ready for cooling, filtration, and filling without the risk of rework or inconsistency.
Batch vs Continuous Production
The choice between batch and continuous syrup production shapes not only workflow but also cost, product consistency, and scalability. Each approach offers distinct benefits depending on production goals, facility capacity, and the diversity of products being made.
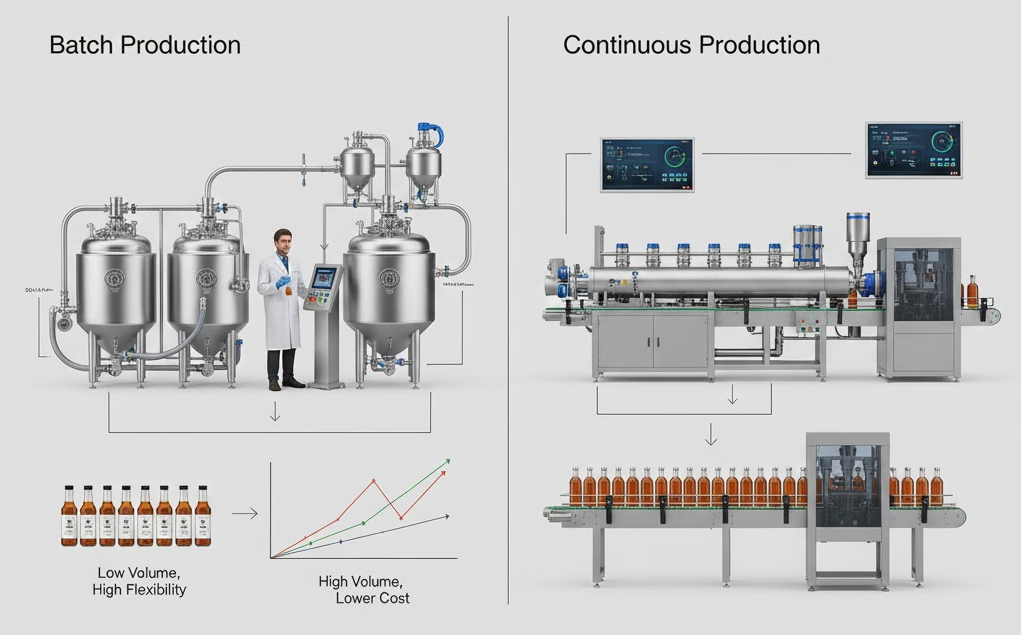
- Batch Production – Ideal for small to medium runs, seasonal flavors, or R&D work. Each batch can be tested and adjusted individually, giving operators full control over Brix, viscosity, and pH. While this method offers flexibility and precise quality control, it requires more labor and longer production cycles.
- Continuous Production – Suited for large-scale, consistent syrup manufacturing. Ingredients flow through continuous mixers, homogenizers, and heat exchangers in one steady process. It delivers higher efficiency and lower operating costs but offers less flexibility when switching between recipes or flavor variants.
In practice, many producers adopt a hybrid strategy — using batch systems for specialty or seasonal products and continuous systems for core SKUs.
Beverage and pharmaceutical manufacturers often favor continuous lines for their cost-efficiency and process control, while artisan and niche brands rely on batch systems for their adaptability and creative freedom.
Recommended Reading: Cartoning Machine vs Case Packer – King Pack Machinery
Cooling and Conditioning
After mixing, the syrup must be cooled to remove residual heat and stabilize the solution before filling. This step allows dissolved gases to escape, which helps prevent foaming and oxidation. Most facilities use jacketed cooling tanks or plate heat exchangers to achieve controlled temperature reduction without altering syrup consistency.

Cooling also lowers microbial growth risk and helps the syrup settle naturally. Conditioning follows this process, ensuring uniform viscosity, balanced sweetness, and a smooth texture with no phase separation. The result is a stable, clear syrup ready for filtration and precise filling in the next stage.
Filtering and Quality Control
Filtration is the final safeguard before syrup enters the filling stage. It removes undissolved particles, air bubbles, and potential microbial contaminants that could affect clarity or shelf stability. Food and pharmaceutical producers typically use sanitary filters in the 0.2–1 μm range to meet hygiene and safety standards without altering flavor or viscosity.

Inline sampling points are used to test clarity, Brix level, pH, and microbial count in real time. This step ensures every batch meets required quality parameters before packaging. Once filling begins, there’s no way to correct contamination or solid residue issues, making effective filtration and quality control essential to maintaining product safety and consistency.
Filling and Packaging of Syrup
After preparation, syrup needs to be packaged efficiently and safely to maintain quality, avoid contamination, and enable transport.
Pre-filling Container Preparation and Sterilization
Before filling, containers must be completely clean and sterile to maintain syrup quality and safety. Both glass and plastic bottles are usually rinsed with sterile water or a sanitizing agent to remove dust or residues.
In pharmaceutical syrup production, containers often go through pre-sterilization using autoclaves or approved chemical sterilants. This step ensures that no microbial contamination occurs during filling. Food-grade syrup lines typically use CIP (Clean-in-Place) systems that sanitize pipes, filling nozzles, and contact parts automatically.
Maintaining container closure integrity (CCI) and overall hygiene reduces contamination risk and supports consistent shelf life. Clean, sterile containers are the foundation of safe and reliable syrup packaging, especially for sensitive or high-purity products.
Recommended Reading: Hand Sanitizer Filling Machine Guide: Working, Types, and Buying Tips – King Pack Machinery
Syrup Filling Machines and Accuracy
For viscous products like syrups and oral suspensions, a piston filler system provides reliable, precise dosing. Modern pneumatic syrup filling machines and Pharmec Oral Liquid / Syrup Filling Lines can handle 6–20 filling nozzles, allowing capacity to scale with production demands. Filling volumes range from 50 ml to 1,000 ml, and mid-to-large fills (500–1,000 ml) maintain filling accuracy of ±0.3%, reducing waste while keeping product consistent.
Constructed with stainless steel 316L and incorporating pneumatic components and PLC control systems, these machines meet food industry hygiene standards. Volumetric based filling ensures even distribution, and integrated Teflon pistons and stainless steel plunger pumps maintain product integrity across multiple filling stations.
Capping, Sealing, and Labelling
After filling, bottles move along automatic conveyor systems to ROPP capping machines or torque-controlled closures. For traceability and branding, automatic labeling systems or circumference labeling units apply pressure-sensitive or shrink-sleeve labels in-line. These synchronized stages ensure that every bottle leaves the line sealed, labeled, and ready for shipment.
Storage and Transport Considerations
Proper storage is essential to preserve syrup quality. Bottles should be stored in clean, cool, and stable conditions, protected from heat, light, and vibration. During transport, using bottle unscramblers, cooling conveyors, and insulated packaging helps maintain consistency, viscosity, and flavor.
Following these protocols, combined with regular maintenance and ultrasonic washing machines or sterilization tunnels, keeps syrups safe, fresh, and compliant with hygiene standards for distribution.
Recommended Reading: Liquid Filling Machine Manufacturer: Top Manufacturers Worldwide – King Pack Machinery
Quality Assurance & Compliance
To maintain both product quality and regulatory compliance, a syrup line must be equipped with robust quality-assurance systems.
Syrup Quality Metrics (Brix, Viscosity, Clarity)
Three key metrics determine syrup quality:
- Brix – Measures sugar concentration. For medicinal syrups, Brix helps in dosing and stability.
- Viscosity – Affects mouthfeel, pourability, and pump behavior. It’s usually measured using a viscometer or rheometer at defined temperatures.
- Clarity – Important for transparent syrups. Turbidity or cloudiness can indicate poor dissolution, microbial growth, or contamination.
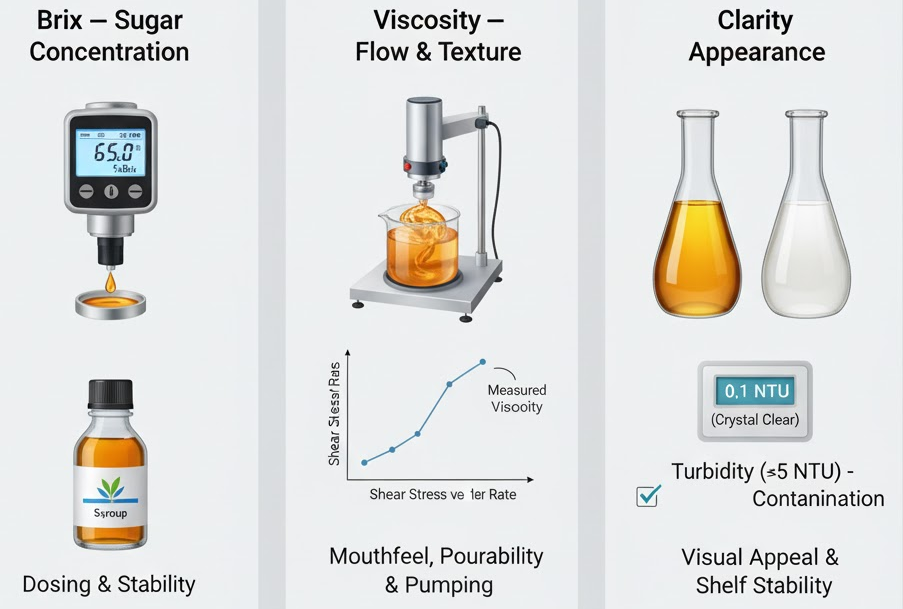
King Pack’s mixing and homogenizing systems help produce stable, consistent syrup in each batch, minimizing deviations in these metrics.
Cleanability, CIP/SIP, and Sanitation
Sanitation is central to syrup production. If equipment isn’t cleaned properly, residual sugar can support microbial growth, leading to spoilage or safety risks.
Best practices:
- Use CIP systems to circulate cleaning solution (alkaline or acidic) through lines without disassembling.
- For higher-risk syrup (e.g., pharmaceutical), use SIP to sterilize tanks and lines with steam.
- Validate cleaning cycles and document them in a quality management system to ensure consistent hygiene.
King Pack machines are designed for CIP compatibility, with sanitary surface finishes, tri-clamp fittings, and hygienic seals — all of which help meet food safety standards.
Regulatory and Food Safety Requirements
Syrup manufacturers must adhere to a variety of food safety regulations. Key considerations include:
- GMP (Good Manufacturing Practices): Ensures consistent and safe production.
- HACCP (Hazard Analysis and Critical Control Points): A system to analyze hazards (e.g., microbial) and define controls (e.g., filtration, temperature).
- Food contact materials: Stainless steel, approved plastics, and seals must comply with local regulations (FDA in the U.S., EFSA in the EU, etc.).
King Pack’s equipment supports these standards through hygienic design, documented materials, and cleanability features.
Challenges & Practical Tips for Syrup Producers
Even with the right machines, syrup manufacturing has real-world challenges. Here’s how to address some of them:
- Foaming during mixing: Use vacuum homogenizers; avoid too high shear speeds initially.
- Scalability: Start with a batch line for R&D, then scale to continuous as volumes grow.
- Flavor degradation: Use nitrogen sparging in tanks to reduce oxidative degradation, especially for delicate fruit or botanical flavors.
- Line downtime: Prioritize CIP/SIP design and invest in automation to reduce the time between runs.
- Quality drift: Regularly calibrate Brix meters, viscometers, and pH probes to maintain consistency.
Conclusion
Syrup manufacturing is more than sugar and water — it’s a careful balance of ingredient control, hygiene, process design, and quality assurance. With the right equipment, producers can ensure stable, clear, and safe syrup at industrial scale.
King Pack offers a powerful suite of solutions for syrup production: from vacuum homogenizing emulsifiers that dissolve and condition syrup efficiently, to piston filling machines that deliver precise, scalable fills. Their modular, food-grade design enables both batch and continuous production while maintaining ease of cleaning and regulatory compliance.
If you’re planning a syrup line or considering upgrading your existing setup, contact King Pack. Our engineering team can work with you to build a customized syrup processing and filling setup that meets your production goals, hygiene standards, and capacity requirements.
