Introduction to Vial Filling Machines
What Is a Vial Filling Machine?
A vial filling machine precisely dispenses pharmaceutical liquids or powders to sterile glass vials. These machines operate in controlled environments to prevent contamination. They form the backbone of injectable drug manufacturing worldwide.

Modern machines process thousands of vials per hour with consistent quality. The global pharmaceutical vial filling machine market reached 1.2 Billion in 2024 and and is forecasted to grow at a CAGR of 9. This growth reflects increasing demand for sterile injectable medications.
Why Efficiency and Accuracy Matter in Pharma Manufacturing
Production costs escalate quickly without efficient filling operations. Wasted product, rejected batches, and downtime all reduce profitability. Accuracy directly affects patient safety and regulatory compliance.
USP requires each injection container to contain sufficient excess volume to allow withdrawal of the labeled drug quantity. Manufacturers must balance minimal waste against extractability requirements. Fill weight accuracy becomes critical at low volumes.
Speed and precision work together. High-speed lines demand reliable performance across millions of cycles. King Pack’s pharmaceutical solutions maintain accuracy even at maximum throughput.
Recommended Reading:
How to Choose the Right Vial Filling Machine for Your Business – King Pack Machinery
Main Types of Vial Filling Machines
Automatic Vial Filling and Stoppering Machine
These integrated systems combine filling and stoppering in continuous operation. Vials move through filling stations where precise volumes get dispensed. Rubber stoppers descend immediately onto filled vials.
The integrated design minimizes contamination risks. Products spend less time exposed to cleanroom environments. Automated stopper placement maintains sterility without human intervention.
Key features of automatic vial filling and stoppering machines include:
| Feature | Benefit |
| Continuous operation | Higher throughput |
| Integrated design | Reduced contamination risk |
| Servo controls | Precise fill volumes |
| Automatic stopper placement | Consistent seal quality |
| Vision inspection | Real-time quality checks |
Production speeds reach 60-400 vials per minute depending on fill volume. Smaller volumes require more precision and run slower. Larger volumes allow faster cycling.
Sterile Vial Filling Machine
Vaccines, biologics, and other injectable pharmaceutical products require a sterile environment throughout the process. King Pack’s Sterile Vial Filling Machine operates in Class 100 clean rooms with HEPA-filtered laminar flow, protecting each vial during aseptic processing. The system meets cGMP requirements and complies with Good Manufacturing Practices, keeping contamination risks minimal at every stage.
The machine’s 316 stainless steel contact parts and rotary piston pump design support full sterilization. Built-in CIP (Clean-In-Place) and SIP (Sterilize-In-Place) functions automate sanitation between batches. Operators can monitor every step through a digital control interface that enhances filling accuracy and production reliability in aseptic liquid filler operations.
Small Vial Filling Machine
Research labs and clinical manufacturing facilities often deal with small batches. The Small Vial Filling Machine handles flexible batch sizes—perfect for R&D or pilot-scale production. Compact construction fits into limited floor space without compromising efficiency or production capacity.
These systems help pharmaceutical manufacturers during early formulation testing or small-scale runs. Quick-change parts and adjustable filling needles allow smooth handling of various vial sizes. Servo drive technology ensures stable operation, while recipe storage keeps parameters consistent for repeat production. This versatility supports cost-effective liquid vial processing and short-run manufacturing.
Vial Filling and Sealing Machine
For complete automation, Vial Filling and Closing Machines combine filling, stoppering, and crimping into one line. The integrated Rotary disc design and CR Series models maintain precise vial positioning during filling and sealing. This setup reduces transfer steps and supports continuous, contamination-free operation.
The system’s crimping stations apply aluminum caps over rubber stoppers with controlled torque. Inspection systems and sensors check for proper closure, fill volume, and stopper placement before vials leave the line. Designed to handle both time pressure filling systems and rotary piston configurations, these machines deliver high filling accuracy and consistent quality—ideal for pharmaceutical manufacturers focused on safety and reliability.
Key Components and Working Principle
Filling System — Volumetric, Peristaltic, or Time-Pressure Mechanisms
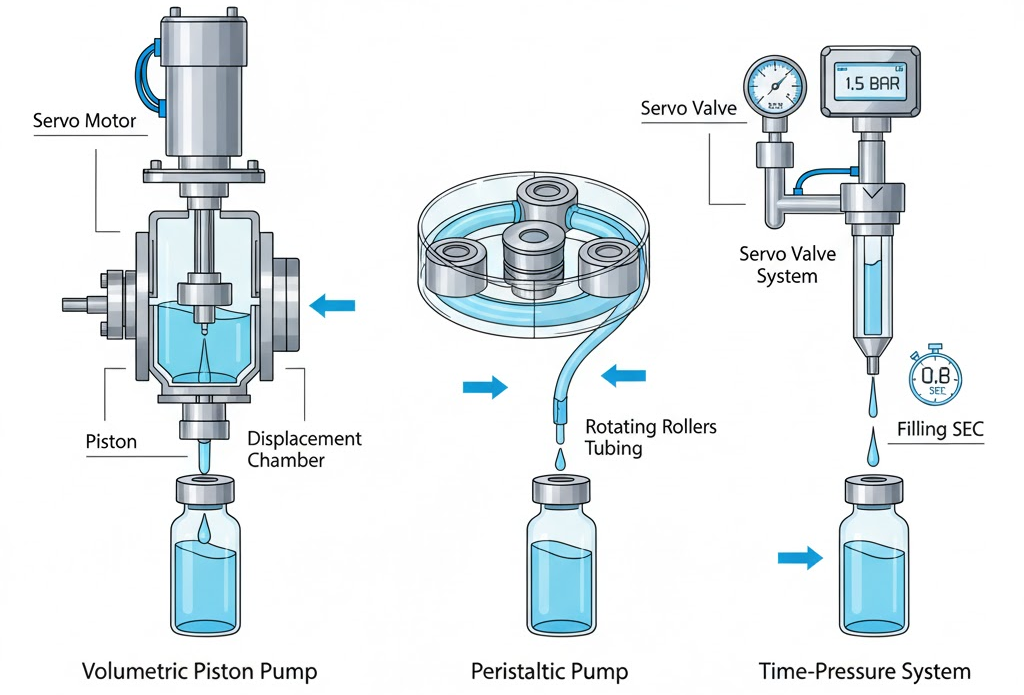
Different filling technologies match different product behaviors. King Pack equips its vial filling systems with advanced options for flexibility, precision, and product integrity.
Volumetric piston filling uses a servo-driven displacement pump. Each piston stroke moves a defined product volume, ensuring consistency even with viscous suspensions or dense liquids. Fill accuracy reaches ±0.5 %, and the system supports in-process control sensors to track dosing deviations in real time.
Peristaltic pump filling involves rotating rollers compressing flexible silicone or Pharmed® tubing. Since the product flows inside the tubing without touching mechanical parts, cleaning is simplified and cross-contamination risk drops sharply. This setup is widely used for biologics and vaccines that are shear-sensitive, as it maintains molecular stability throughout filling.
Time-pressure filling relies on controlled air or nitrogen pressure to displace liquid through the nozzle. The servo valve opens for an exact time, and the pressure curve regulates volume. This suits products with variable viscosity—such as reconstituted antibiotics—where mechanical displacement is less efficient.
Stoppering System — Rubber Stopper Placement Under Sterile Conditions
Once vials are filled, sterile rubber stoppers must be inserted immediately to preserve aseptic conditions. Stoppers are first sterilized through autoclaving or gamma irradiation and stored in sealed tubs. Automated stoppering systems transfer these to sterile bowls inside ISO 5 zones using restricted-access barrier systems (RABS).
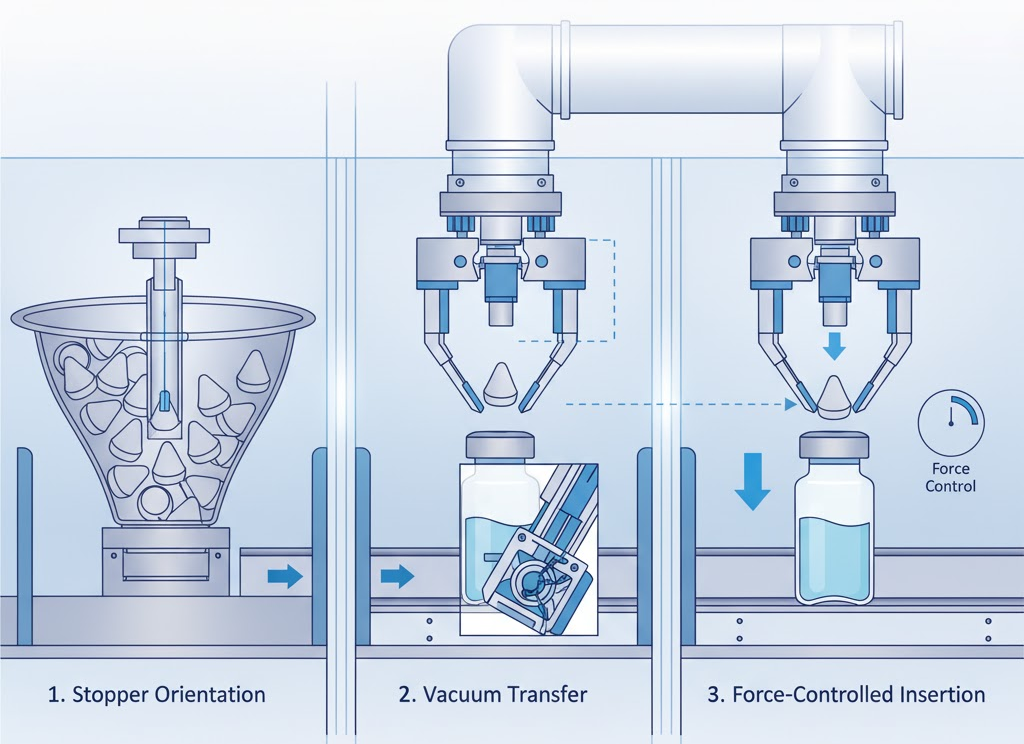
Orientation arms and vibration tracks align stoppers for precise pick-up. Vacuum or mechanical grippers collect individual stoppers and insert them over vial openings in perfect synchrony with conveyor indexing. Force sensors control insertion depth to prevent splashing or pressure buildup inside vials.
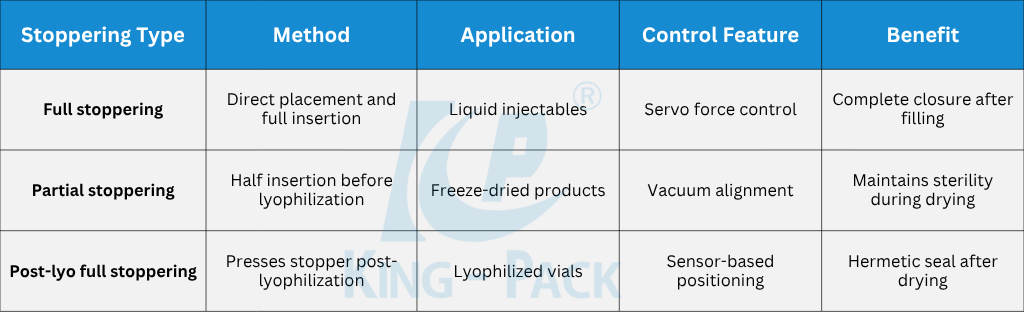
For lyophilized products, the system performs partial stoppering to allow sublimation during freeze-drying. Once lyophilization ends, the stoppers are fully seated using servo presses, forming airtight seals. This transition occurs without removing vials from the sterile enclosure, protecting product sterility until capping.
Capping & Sealing — Secure Closure for Product Integrity
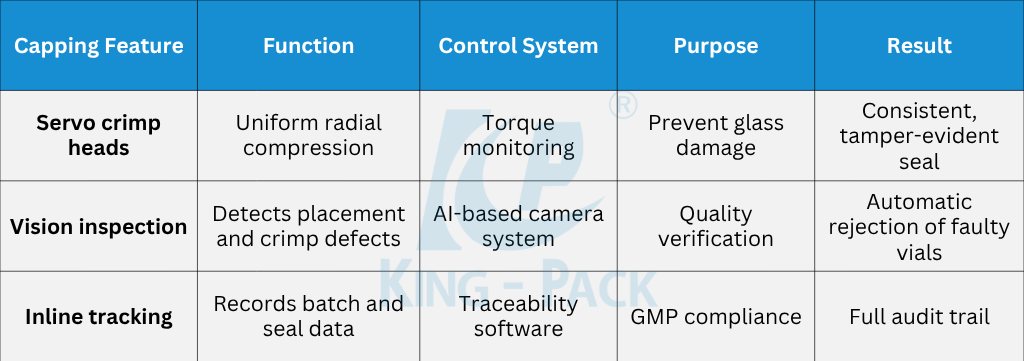
Capping is the final mechanical safeguard for filled vials. Aluminum caps or flip-off seals are positioned over stoppered vials and crimped by rotating or segmental heads. Each crimp station applies uniform radial pressure to fold the aluminum skirt securely around the vial neck.
Servo-controlled torque monitoring ensures consistent compression without damaging glass. A uniform crimp guarantees mechanical integrity and tamper evidence while maintaining sterility. Modern lines can process up to 400 vials per minute depending on size and fill volume.
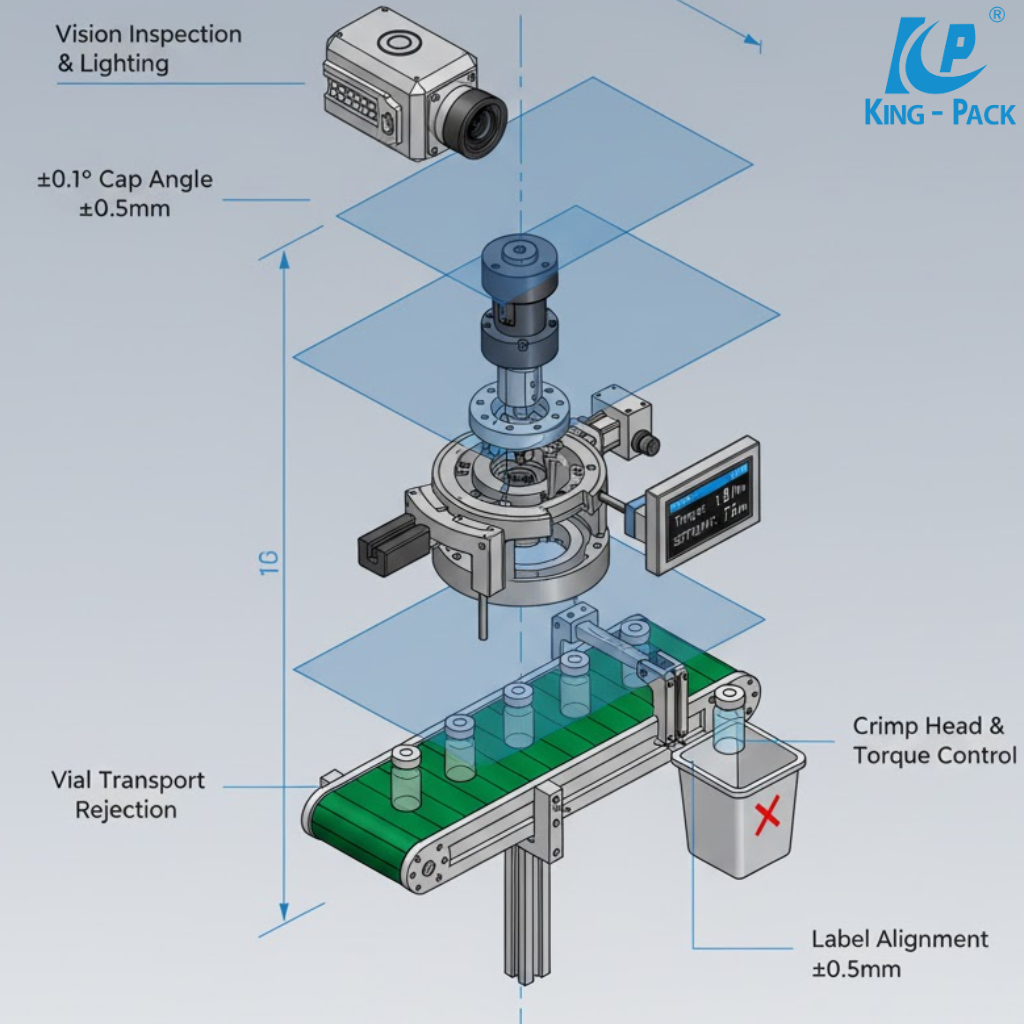
Inline vision inspection verifies correct cap color, placement, and crimp shape. High-resolution cameras detect deformed caps or misalignments, while torque sensors measure sealing strength. Defective units are automatically rejected through pneumatic diverters before packaging.
King Pack integrates these verification systems at multiple stages—filling, stoppering, and sealing. Early fault detection minimizes waste and ensures only compliant units proceed downstream.
Benefits of King Pack Vial Filling Machines
Precision and Consistency Across Every Vial
Fill weight accuracy and precision represent major challenges during aseptic filling operations. King Pack addresses this through advanced servo technology and real-time monitoring. Every vial receives the specified volume within tight tolerances.
Statistical process control tracks performance continuously. Operators receive alerts when drift occurs. Corrective action happens before products go out of specification. This prevents batch rejections and costly rework.
Fully Automatic Operation and High Productivity
Manual filling operations cannot match automated speed. Labor costs multiply when processing millions of vials annually. King Pack’s vial filling machine manufacturers design equipment that runs three shifts with minimal supervision.
Production rates depend on fill volume and product characteristics:
| Fill Volume | Typical Speed |
| 2-5 mL | 200-400 vials/min |
| 5-10 mL | 150-300 vials/min |
| 10-20 mL | 100-200 vials/min |
| 20-50 mL | 60-120 vials/min |
Faster speeds reduce cost per unit. Automated inspection maintains quality without slowing production. This combination maximizes return on investment.
Recommended Reading: How Vial Filling Machines Improve Efficiency and Accuracy in Liquid Filling – King Pack Machinery
Sterile Design and GMP Compliance
FDA requires proper sterile gowning, masks, caps, and shoe coverings in filling rooms. Equipment design must support these requirements. King Pack builds machines that minimize operator intervention in sterile zones.
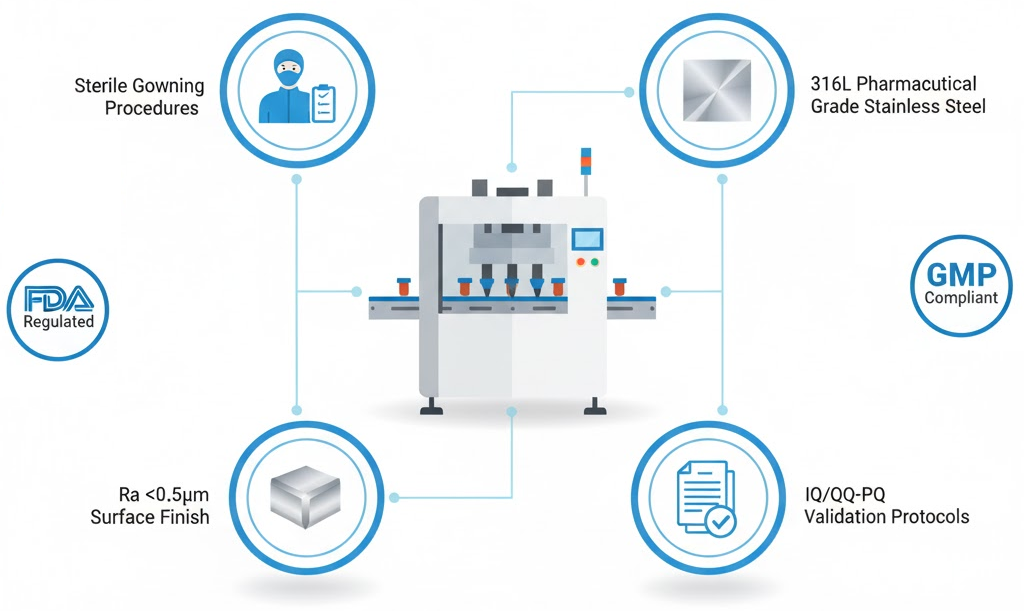
All product-contact surfaces use pharmaceutical-grade stainless steel. Welds get polished to eliminate crevices where contamination might hide. Gaskets and seals meet USP Class VI requirements.
Documentation packages include validation protocols. Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) support regulatory submissions. This accelerates approval for new facilities.
Customizable for Liquid, Powder, or Injectable Products
Different products demand different handling. Liquid formulations flow easily through standard filling systems. Powders require specialized dosing mechanisms. King Pack customizes equipment for specific applications.
Lyophilized products need vial processing through freeze-dryers. Machines accommodate partially stoppered vials entering and exiting lyophilization chambers. After drying, final stoppering and capping complete the packaging.
Temperature-sensitive biologics may require chilled filling zones. Heating systems handle viscous products flowing better when warm. King Pack engineers solutions matching product requirements exactly.
How to Choose the Right Machine for Your Business
Identify Production Scale: Lab, Medium, or Mass Production
Before choosing a vial filling system, the first step is understanding your production scale. Small labs and R&D centers usually process fewer than 10,000 vials a year. In these settings, flexibility matters more than speed. A compact or bench-top vial filling machine is ideal—it saves space, supports frequent changeovers, and keeps upfront costs low.
Medium-scale manufacturers handle anywhere between 100,000 and 1 million vials annually. These operations often move from manual to semi-automatic equipment. Operators still load or unload vials manually, but the filling and stoppering processes run automatically. This balance improves efficiency without requiring the full investment of a large-scale line.
For mass production exceeding 10 million vials annually, automation becomes essential. Fully automatic lines cover everything—from washing and depyrogenation to filling, stoppering, inspection, and capping. These turnkey systems are designed to maintain high throughput while meeting strict GMP and aseptic standards.
| Production Scale | Vial Output (Annual) | Machine Type | Automation Level | Ideal For |
| Lab-scale | <10,000 | Small vial filling machine | Manual or semi-auto | R&D, pilot studies |
| Medium-scale | 100,000–1,000,000 | Semi-automatic filling and stoppering | Partial | Regional manufacturers |
| Mass-scale | >10,000,000 | Fully automatic integrated line | Full | Large pharmaceutical plants |
Select Based on Product Type (Liquid vs. Powder)
Your product formulation plays a big role in choosing the right filling technology. Liquid injectables—like vaccines, suspensions, or saline-based drugs—demand smooth, consistent flow control. The viscosity determines which pump works best: piston fillers handle thicker solutions, while peristaltic pumps are perfect for delicate biologics that need gentle handling.
For powder formulations, such as antibiotics or freeze-dried drugs, precision dosing is key. Powder filling machines often use auger or vacuum systems to control volume and prevent airborne contamination. Operating in a controlled environment ensures dry powders don’t absorb moisture before sealing.
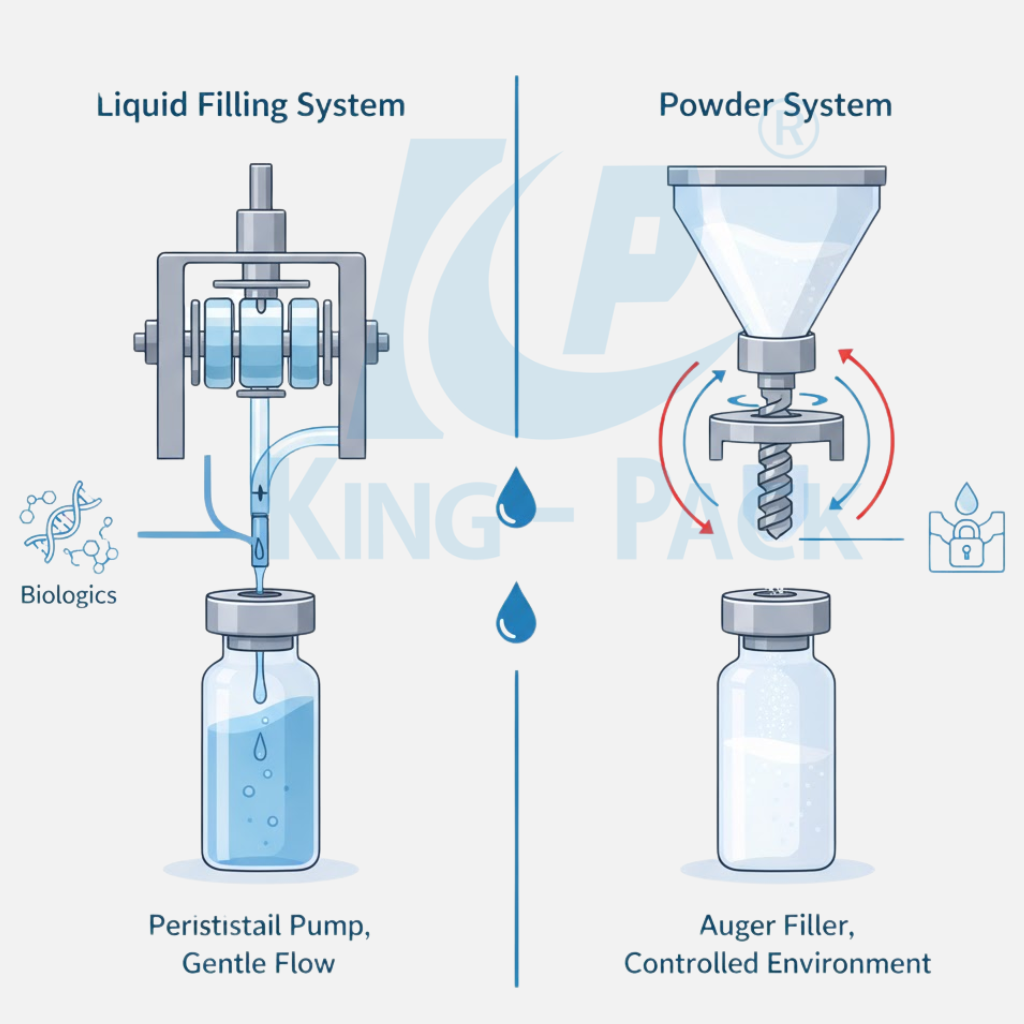
Recent reports show that biologics make up nearly half (48.6%) of the global vial market. These high-value formulations require extreme care during filling. Systems designed for biologics use closed paths and low-shear flow to protect protein stability—preventing aggregation or denaturation.
| Product Type | Recommended Filling Method | Typical Application | Handling Requirement |
| Liquid | Piston or peristaltic pump filling | Vaccines, injectables | Controlled laminar flow, accurate dosing |
| Powder | Auger or vacuum filling | Antibiotics, lyophilized drugs | Dry environment, precise metering |
| Biologic | Peristaltic or time-pressure filling | Protein-based products | Gentle flow, low shear stress |
Consider Budget, Maintenance, and Integration Needs
The right machine should balance performance and long-term value. The initial price is only one part of the equation. Ongoing expenses—like labor, maintenance, utilities, and spare parts—contribute to the total cost of ownership. Evaluating these helps prevent unexpected budget strain later on.
Maintenance also depends on the level of automation and system complexity. A simpler semi-automatic unit might only require basic cleaning and lubrication, while a full aseptic line involves calibration and software diagnostics. Manufacturers that offer spare parts support, operator training, and preventive maintenance plans reduce downtime and extend machine lifespan.
Integration is another key factor. Standalone vial fillers can operate independently in smaller setups, while larger facilities may prefer fully integrated lines that connect washing, filling, and inspection units under a single control system. Seamless communication between machines boosts line efficiency and traceability.
| Factor | What to Evaluate | Why It Matters |
| Budget | Purchase + operating cost | Determines long-term ROI |
| Maintenance | Spare parts, training, downtime | Affects uptime and reliability |
| Integration | Compatibility with current line | Reduces transition time and errors |
King Pack Consultation: Tailored Solutions for Your Line
Every facility has unique production goals and constraints. Floor space, vial size, output volume, and product sensitivity all play a part in designing the right system. That’s why personalized consultation helps streamline decision-making.
King Pack’s engineers start with a requirement analysis, studying your formulations, batch sizes, and cleanroom setup. Based on this, they recommend machine configurations that fit both your budget and regulatory standards.
Before shipment, each system goes through Factory Acceptance Testing (FAT), where clients can verify that the equipment meets every performance benchmark—from fill accuracy to line speed. This hands-on approach ensures smooth installation and fast startup once the machine reaches your facility.
| Consultation Stage | Purpose | Outcome |
| Requirement analysis | Assess production needs | Tailored equipment selection |
| System configuration | Match process and compliance goals | Optimized line design |
| FAT validation | Test machine before delivery | Proven performance and reliability |
Why King Pack Is a Trusted Vial Filling Machine Manufacturer
Premium quality doesn’t require premium prices. King Pack manufactures to European engineering standards while maintaining cost advantages. This provides customers excellent value propositions.
King Pack’s quality assurance process includes mechanical stress testing, fill accuracy checks, and electrical safety verification. Before shipment, each unit undergoes a full performance trial replicating real production conditions. This guarantees stable operation once the equipment reaches your facility.
By combining advanced engineering with in-house manufacturing, King Pack delivers machines that rival European standards but at far more competitive pricing. Customers benefit from reduced lead times, flexible customization, and long-term cost savings without compromising quality or reliability.
Conclusion
King Pack delivers end-to-end vial filling and processing systems that meet modern pharmaceutical production demands. Whether you’re launching a new injectable product or expanding an existing line, King Pack’s engineering team can design a setup that fits your production scale, layout, and regulatory goals. We provide testing, installation, and lifetime support to keep your operations efficient and compliant.
Contact King Pack today to discuss your vial filling requirements and get a custom-built solution that delivers precision, productivity, and long-term value for your pharmaceutical business.
Recommended Reading: World Top 10 Vial Filling Machine Manufacturers – King Pack Global Pharma Solutions – King Pack Machinery