Pharmaceutical companies face strict requirements for sterile production. Each vial must be filled with precision, sealed without exposure, and traceable through every batch. Meeting these standards requires equipment that performs flawlessly under cleanroom conditions.
New-generation vial filling systems are designed with this in mind. Compact layouts, smart controls, and automated quality checks now make aseptic production more efficient than ever. In this article, we’ll look at how automated vial filling and processing lines meet the growing demands of modern pharmaceutical manufacturing.
Challenges & Objectives in Vial Processing
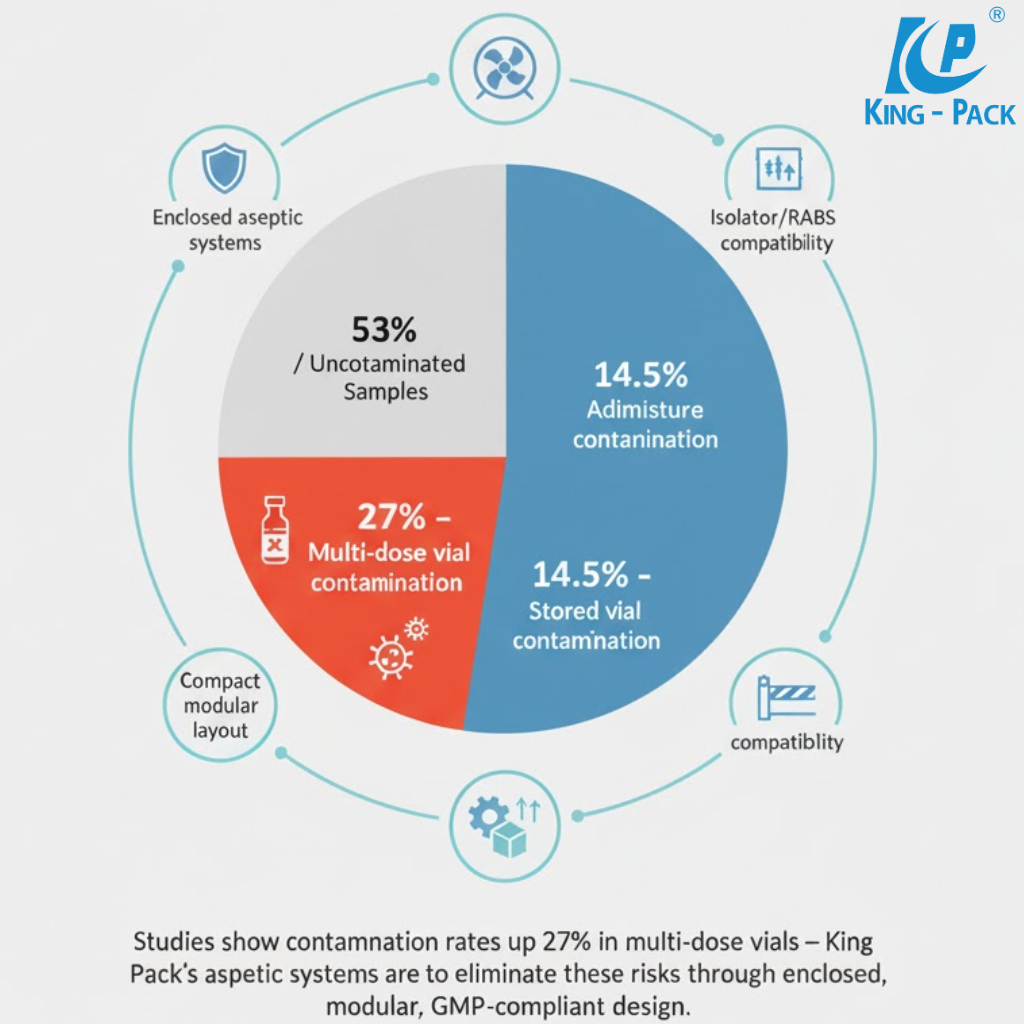
Vial contamination remains one of the biggest challenges in pharmaceutical manufacturing. Even a small amount of microbial or particulate contamination can compromise injectable drugs. Studies show contamination rates reaching up to 14.5% in admixtures and nearly 27% in multi-dose vials.
That’s why today’s manufacturers aim for zero contamination. King Pack’s automated vial filling machines and aseptic filling machines are designed to meet this standard by combining precision, sterility, and reliability.
Each Container Filling System operates inside a sterile tunnel or laminar airflow hood, maintaining a controlled environment throughout the process. The filling needle, rotary piston, and valve assemblies work together to maintain accurate dosing with minimal product contact.
Enclosed systems with aseptic liquid fillers prevent airborne particles from entering the critical zone. From washing to filling, plugging and capping, every stage is handled under monitored cGMP requirements using filtered laminar airflow protection to reduce microbial presence.
Space efficiency is another key concern in bio-safety cabinets and Class 100 cleanroom environments. Pharmaceutical cleanrooms often have strict zoning, so equipment needs to combine multiple operations within compact footprints. King Pack integrates washing, filling, stoppering machines, and capping functions into one streamlined line.
By minimizing transfer points between modules, contamination risks drop significantly while improving operational flow. Modern vial processing lines include components such as servo technology-driven dosing systems, Inline Volumetric Fillers, and torque sensors that enhance accuracy and repeatability. Some configurations use vibratory bowls for component feeding or quickconnect systems for faster changeovers between batches.
Machines equipped with touch screen HMI controls simplify operation and parameter adjustment, while Hot foil code printing, ink jet print heads, and labeling systems manage downstream packaging without slowing production.
Designing for Aseptic Isolator or RABS (Restricted Access Barrier System) compatibility is also crucial. These systems separate the operator from the sterile zone, greatly reducing human contact and the risk of medication errors. Combined with automatic syringe filling processes, Cartridge Filling Systems, and advanced product dispensing systems, King Pack’s vial lines support long production runs with minimal manual handling.
This improves both safety and cost savings through reduced downtime and inventory management precision. Since drains and sinks are restricted in Grade A and B areas, King Pack machines include closed-loop waste handling and smooth, stainless steel surfaces that simplify cleaning.
Each system complies with good manufacturing practices and ISO 14644 cleanroom classifications. Together, these features help pharmaceutical manufacturers maintain sterile operations, reduce downstream processing contamination, and maximize efficiency in limited spaces.
Recommended Reading: Maintenance Tips for Vial Filling Machines to Ensure Longevity – King Pack Machinery
Core Modules of the Vial Processing Line
Washing & Depyrogenation (Container Decontamination)
Vials used in pharmaceutical production often contain traces of glass dust, lubricants, or manufacturing residues. These contaminants can compromise drug purity if not removed properly. Washing is the first step in vial preparation.
Purified water or Water for Injection (WFI) sprays both inside and outside each vial, dislodging particles and residues. Multi-stage washers combine ultrasonic cleaning, pressure rinsing, and filtered air drying to achieve consistent results.
After washing, depyrogenation removes endotoxins that could cause fever or immune reactions in patients. This process uses high heat to destroy pyrogenic substances on the vial surface.
Depyrogenation tunnels maintain stable temperatures between 250–350°C for 30–60 minutes.
According to FDA and WHO guidelines, these conditions achieve a minimum 3-log reduction in endotoxin levels, ensuring vials are sterile and safe for aseptic filling.
King Pack’s integrated washing and depyrogenation systems minimize handling between stages. Vials move automatically from the washer into the depyrogenation tunnel under a protected environment, preventing recontamination.
The entire line operates under validated control parameters and records every batch cycle for traceability. This ensures compliance with GMP and ISO standards while maintaining high production throughput.
Key washing parameters:
| Parameter | Typical Range |
| WFI Temperature | 70-90°C |
| Depyrogenation Temp | 250-350°C |
| Tunnel Transit Time | 30-60 minutes |
| Log Reduction | ≥3 log |
Aseptic Filling & Stoppering (Liquid / Powder Filling)
Filling is one of the most critical operations in sterile drug manufacturing. It must be performed under Grade A aseptic conditions, equivalent to ISO Class 5 air cleanliness. These areas are dedicated to high-risk activities such as vial filling, stopper bowl handling, and product transfer.
Laminar airflow systems deliver a steady stream of HEPA-filtered air, maintaining particle-free zones and protecting the open vials during filling.

Different pharmaceutical formulations require different filling mechanisms. King Pack’s vial filling systems are designed for flexibility, precision, and contamination control.
- Piston filling – Ensures high accuracy for viscous and dense liquids.
- Peristaltic pump filling – Suitable for biologics and vaccines, preventing shear stress.
- Time-pressure filling – Adjusts to liquids with varying viscosities.
- Powder filling – Supports lyophilized and dry pharmaceutical products.
Each filling technology achieves ±0.5% or better volume accuracy, driven by servo motors that ensure smooth, consistent performance. Real-time sensors detect any irregularities in flow or volume and trigger alerts instantly. This minimizes product waste and supports GMP validation.
Once filled, automatic stoppering takes place immediately within the sterile zone. Rubber stoppers are placed without using vacuum systems, reducing the risk of external air entry. The entire stoppering process occurs under laminar airflow to prevent airborne contamination. This method protects product sterility until capping and sealing.
| Parameter | Typical Range / Feature | Description |
| Cleanroom Classification | Grade A / ISO 5 | Maintains sterile conditions during filling |
| Fill Accuracy | ±0.5% or better | Ensures dosing precision and product uniformity |
| Filling Range | 0.5 mL – 100 mL | Adjustable depending on vial size and formulation |
| Filling Types | Liquid / Powder | Supports injectable solutions and lyophilized drugs |
| Pump Options | Piston, Peristaltic, Time-Pressure | Flexible for viscosity and sensitivity needs |
| Stoppering Speed | Up to 200 vials/min | High-speed operation with sterile handling |
| Monitoring | Real-time sensors | Detect fill deviations and trigger alarms |
| Airflow | Laminar, HEPA-filtered | Prevents airborne contamination |
This integrated aseptic filling and stoppering system maintains consistent sterility, reduces manual contact, and supports compliance with GMP and cGMP standards. It ensures every vial is accurately filled, securely sealed, and ready for downstream processing.
Recommended Reading: A Detailed Guide to Vial Filling Machine Types – Principles and Selection | King Pack – King Pack Machinery
Capping & Closure (Aluminum, Flip-Off, Bio-Set, etc.)
The capping stage provides the final mechanical seal that protects sterile vials from contamination and leakage. After stoppering, aluminum caps are crimped securely over the rubber stoppers using controlled mechanical pressure.
This process not only ensures a tight seal but also protects the integrity of the drug product during transport and storage. Each vial type may require a specific closure design depending on its intended use and regulatory requirements.
Common closure styles include:
- Aluminum flip-off caps – Allow easy access while maintaining tamper evidence.
- Tear-off caps – Used for products requiring complete removal before use.
- Color-coded caps – Aid in quick batch or formulation identification.
- Tamper-evident seals – Provide visible proof of product integrity.
Modern vial capping systems use torque control and sensor-based feedback to ensure every cap meets proper sealing standards. Vision inspection cameras verify crimp quality and detect missing or misaligned caps with 100% accuracy. Any vial that doesn’t meet specification is automatically rejected to maintain GMP compliance.
King Pack’s automatic vial capping machines integrate seamlessly with upstream filling and stoppering units. Their synchronized operation maintains line balance and avoids bottlenecks. Quick-change tooling supports multiple vial diameters and closure types, reducing downtime between product runs.
| Parameter | Typical Range / Feature | Description |
| Cap Type | Aluminum, Flip-Off, Tear-Off, Bio-Set | Compatible with standard and specialty closures |
| Cap Diameter | 13 mm – 32 mm | Adjustable via quick-change components |
| Crimp Force | Servo-controlled | Ensures consistent seal integrity |
| Inspection Rate | 100% | Vision system checks crimp and cap presence |
| Line Speed | Up to 200 vials/min | Depends on vial size and closure type |
| Reject Mechanism | Automatic ejection | Removes improperly capped or missing caps |
With these systems, pharmaceutical manufacturers achieve reliable, repeatable sealing that aligns with international GMP and FDA standards. This stage finalizes product security and prepares vials for labeling and final packaging.
Labelling (Self-Adhesive Label Application, Vision Inspection)
Labels carry critical product information. Regulatory requirements demand accuracy and readability. Automated systems apply labels consistently.
King Pack’s vial labelling machine features:
- Precise label placement (±0.5mm tolerance)
- Multiple label stations for front/back application
- Barcode verification systems
- Vision inspection for quality control
Inspection systems verify:
- Label presence
- Correct orientation
- Print quality and readability
- Expiration date accuracy
Rejection mechanisms remove defective units automatically. Traceability systems track every vial through production.
Advanced Line & System Solutions
Integrated Turnkey Vial Filling & Capping Lines
Complete production lines eliminate integration challenges. King Pack provides end-to-end solutions from washing through inspection.
A typical automatic vial filling system includes:
- Vial washing and depyrogenation
- Aseptic filling and stoppering
- Visual inspection
- Capping and crimping
- Labeling and coding
- Final inspection
- Packaging
Single-source responsibility simplifies validation. All components communicate through unified control systems. This reduces complexity and improves reliability.
Flexible Configurations for Liquids, Powders, and Mixed Use
Modern pharmaceutical vial filling line equipment handles multiple product types. Changeover capabilities reduce downtime between batches.
Configuration options:
| Application | Technology | Speed Range |
| Liquid sterile filling | Piston/Peristaltic | 60-400 vials/min |
| Powder/Lyophilized | Vacuum dosing | 40-200 vials/min |
| Suspension products | Time-pressure | 80-300 vials/min |
| High viscosity | Heated pumps | 50-150 vials/min |
Tool-free format changes accommodate various vial sizes. Recipe management stores parameters for each product. Operators switch formulations through touchscreen controls.
Automation, Modular Design, and Minimal Human Intervention
Automated systems reduce contamination risks. Grade B areas require particle monitoring systems with alarms when limits exceed specifications. Automation minimizes operator presence in critical zones.
Modular construction allows phased expansion. Manufacturers start with core filling capabilities. They add inspection, packaging, and downstream equipment as production grows.
Remote monitoring provides real-time performance data. Predictive maintenance identifies issues before failures. This maximizes uptime and production efficiency.
Material Handling & Loading Systems
Tray Loading and Unloading (Automatic Systems)

Vials move through production in specialized trays or nests. Automated loading eliminates manual handling in sterile zones.
Tray systems offer several advantages:
- Consistent vial orientation
- Protection during transport
- Compatible with freeze-drying
- Simplified tracking
Robotic systems load empty vials at line start. After processing, unloading systems transfer filled vials to secondary packaging. Vision systems verify tray loading accuracy.
Vial Feeding / Buffering & Integration with Other Equipment
Buffer zones prevent line stoppages. Accumulation conveyors store product between processing stages. This maintains continuous flow despite speed variations.
King Pack’s sterile vial filling solutions include optimized buffering. Proper spacing prevents vial collisions. Star wheels and timing screws provide precise positioning.
Integration with upstream equipment creates seamless workflows:
- Filling machines feed directly to inspection
- Approved vials transfer to capping
- Rejected units route to quality review
- Finished products move to cartoning
Recommended Reading: World Top 10 Vial Filling Machine Manufacturers – King Pack Global Pharma Solutions – King Pack Machinery
Supporting Technologies (Sterilization, Drying, etc.)
Depyrogenation Tunnels & Dry Heat Sterilizers
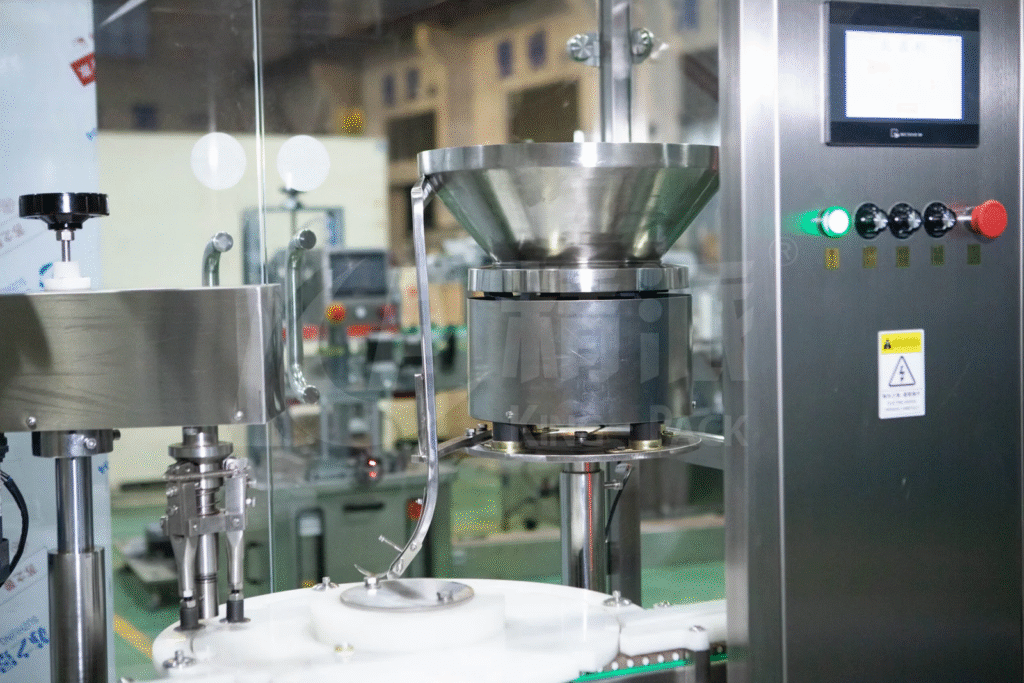
Depyrogenation tunnels use dry heat for container sterilization. Temperature zones gradually heat vials to prevent thermal shock. Peak temperatures destroy endotoxins effectively.
Tunnel zone configuration:
| Zone | Function | Temperature |
| Preheat | Gradual warming | 100-150°C |
| Sterilization | Endotoxin destruction | 250-350°C |
| Cooling | Controlled reduction | 150-ambient |
Tunnel validation confirms heat distribution. Temperature mapping identifies cold spots. Biological indicators verify sterilization effectiveness.
Drying, Heat Treatment, and Thermal Management
Moisture elimination prevents microbial growth. Drying systems use HEPA-filtered air in controlled environments. Temperature and humidity monitoring confirms specifications.
Heat treatment serves multiple purposes:
- Container depyrogenation
- Component sterilization
- Product lyophilization
- Terminal sterilization
Thermal management maintains product stability. Cold chain requirements demand precise temperature control. King Pack equipment includes validated thermal systems.
Recommended Reading: How to Choose the Right Vial Filling Machine for Your Business – King Pack Machinery
Choosing King Pack vs Others
Performance under GMP / cGMP Requirements
GMP facilities must maintain Grade A through D classifications based on operation criticality. King Pack designs all vial filling machine manufacturers equipment for GMP compliance.
Documentation packages include:
- Design Qualification (DQ)
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
Validation support accelerates regulatory approval. Factory Acceptance Testing (FAT) confirms performance before shipment. Site Acceptance Testing (SAT) verifies proper installation.
Cost Efficiency, After-Sales Support, Customization
Investment analysis considers total cost of ownership. Energy-efficient components reduce operating expenses. Reliable operation minimizes costly downtime.
King Pack provides comprehensive support:
- Installation and commissioning services
- Operator training programs
- 24/7 technical assistance
- Spare parts availability
- Preventive maintenance planning
Customization addresses unique production requirements. Equipment specifications match exact product characteristics. Bottle sizes, fill volumes, and production speeds all factor into designs.
Compatibility with Isolators, RABS, or Cleanrooms
Grade A operations often use isolators or laminar flow cabinets within Grade B backgrounds. King Pack’s pharmaceutical vial filling line equipment integrates with all barrier technologies.
System compatibility:
- Open cleanroom installations
- Passive RABS configurations
- Active RABS with dedicated airflow
- Full isolator systems with H2O2 decontamination
- Hybrid combinations
Pressure differentials maintain directional airflow. Continuous monitoring confirms environmental parameters. Alarm systems alert operators to excursions immediately.
Recommended Reading: Everything You Need to Know About Vial Packaging Machines – King Pack Machinery
Conclusion
King Pack delivers complete vial filling and processing solutions. From washing through labeling, our systems handle every production stage. We design systems for both small-scale R&D labs and large pharmaceutical manufacturers, with full flexibility for vial sizes, fill volumes, and production speeds.
Partner with King Pack today to develop a vial filling line that supports your growth and upholds pharmaceutical precision at every stage.