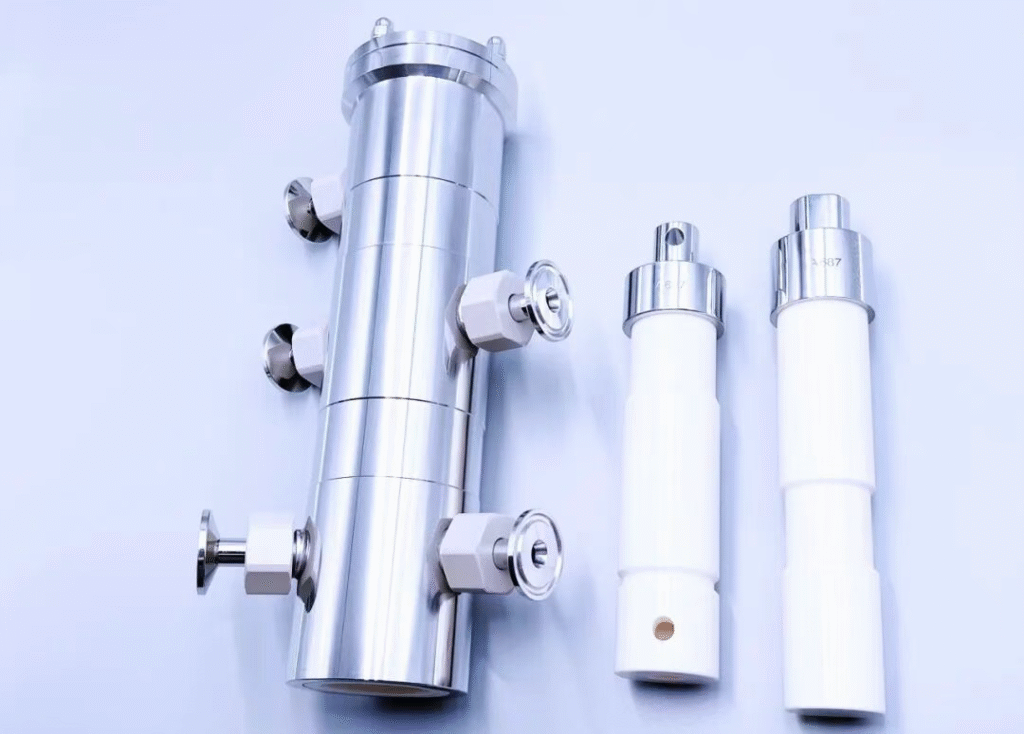
In high-end aseptic filling scenarios for biologics, vaccines, and other sterile products, the thoroughness and timeliness of the sterilization process directly determine production efficiency. King Pack is a subsidiary of Truking Technology, a leading Chinese pharmaceutical equipment enterprise. The S-Type CIP-SIP Ceramic Pump developed by King Pack has become a crucial solution to industry pain points, offering a standardized In-Line Cleaning and Sterilization process and extreme efficiency benefits. Specifically, it provides significant advantages over traditional pumps in terms of sterilization operational procedures and, most importantly, sterilization efficiency.
✨ Part.1 S-Type CIP-SIP Ceramic Pump In-Line Cleaning and Sterilization Process
The S-Type pump adopts the design concept of “Clean-In-Place (CIP) + “Sterilize-In-Place (SIP),” allowing for In-Line Cleaning and Sterilization without disassembly, meeting GMP requirements for sterile production. The process is divided into core stages: CIP (Pre-rinse, Pressure Holding, Alkaline Wash, Final Rinse) and SIP (Heating, Sterilization, Pressure Holding), with each stage operating through a set procedure:
01 // CIP (Clean-in-Place)
In-Line Cleaning (CIP) is an automated process where the core components—piping, ceramic pump, and buffer tank—remain assembled. Pre-set programs (using specific temperature and pressure) and cleaning agents (such as alkali or Purified Water) are used to automatically clean all product-contact surfaces inside the equipment, removing residual materials, soil, and microorganisms to achieve the required cleanliness standard.
- Pre-Rinse: At the filling station, the filling pump’s action dilutes the residual liquid in the pipes, reducing residue and cutting down on final rinse time. (Medium: Purified Water or Water for Injection (WFI))
- Pressure Holding: At the cleaning station, compressed air is used to maintain a pressure greater than required for the entire CIP/SIP process for a period of time, ensuring no leaks in the piping. (Medium: Sterile Compressed Air)
- Alkaline Wash (Process Dependent): The buffer tank, distributor, ceramic pump, and piping are soaked for 30 minutes using an alkaline solution (e.g., NaOH solution). (Medium: 1% to 2% Concentration Alkaline Solution)
- Final Rinse: At the cleaning station, WFI and clean compressed air are supplied by the CIP station to rinse the piping and filling pump according to the cleaning process. The conductivity value must be monitored, with a reading not exceeding $2.7\mu s/cm$ (WFI conductivity value). (Medium: Water for Injection and Clean Compressed Air)
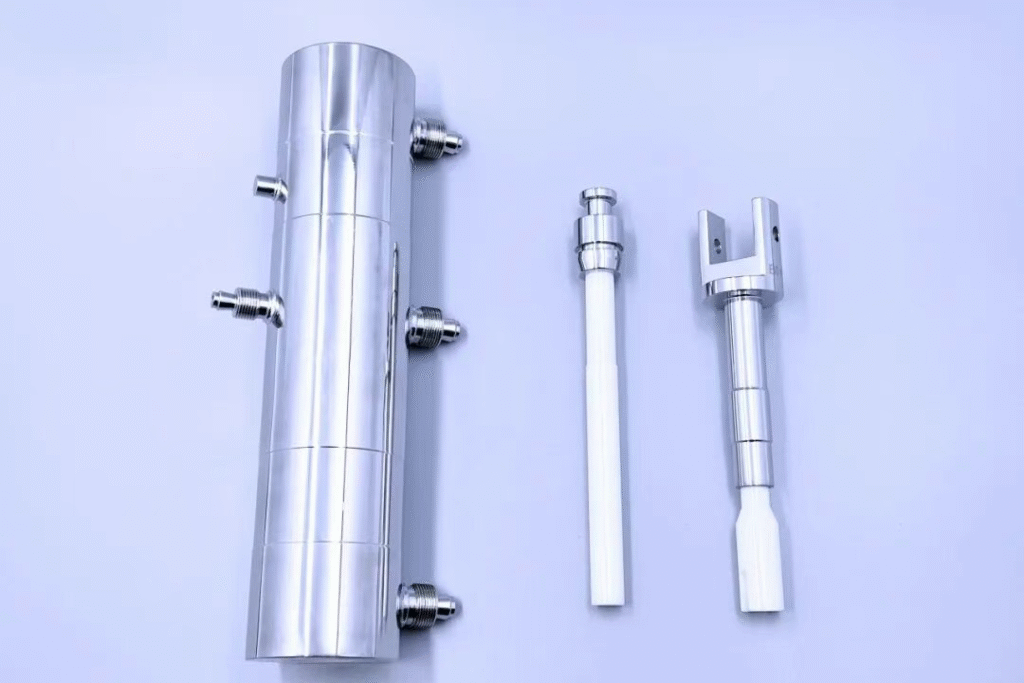
02 // SIP (Sterilize-in-Place)
In-Line Sterilization (SIP) is performed after CIP completion, also without equipment disassembly. High-temperature, high-pressure sterilization media (such as Saturated Pure Steam) are used to sterilize the areas contacting sterile materials (e.g., the filling machine’s ceramic pump and buffer tank), ensuring the equipment reaches a “sterile state” to meet regulatory requirements (e.g., FDA, GMP) for aseptic production.
- Heating: Saturated pure steam flows through the entire pipeline, heating and maintaining a constant temperature of 123°C through heat exchange; (Medium: Saturated pure steam)
- Sterilization: Maintaining a constant temperature of 123°C for 30 minutes kills microorganisms within the pipeline, achieving sterilization; (Medium: Saturated pure steam)
- Pressure Holding: Maintain positive pressure throughout the pipeline to prevent external contaminants from backing up into the pipe until the next production run. (Medium: Clean Nitrogen)

📈 Part.2 Efficiency Comparison with Traditional Aseptic Filling Pumps
Traditional aseptic filling pumps, which do not support In-Line Sterilization, require disassembly for separate sterilization. This results in significant time cost and equipment utilization disadvantages compared to the King Pack S-Type CIP-SIP Pump. The specific data comparison is as follows:
| Comparison Metric | King Pack S-Type CIP-SIP Ceramic Pump | Traditional Aseptic Filling Pump | Efficiency Improvement |
| Equipment Downtime Percentage | <5% Sterilization can run concurrently during production breaks | 20%–30% Requires dedicated sterilization period | 75%–83% |
| Changeover Sterilization Efficiency | Supports multi-batch continuous sterilization Changeover interval <5 hours | Requires manual disassembly and re-sterilization for each changeover Interval ≥ 8 hours | 60% |
| Annual Maintenance Downtime | <5 days No disassembly wear; ceramic components have long lifespan | 15–20 days Frequent disassembly causes component wear | 67%–75% |
| Personnel Operation Intervention Frequency | Only requires program activation Fully automated process | Disassembly, cleaning, assembly, and sterilization all require manual operation | Reduced by over 90% |
King Pack Ceramic Pumps significantly outperform traditional pumps in sterilization efficiency and other aspects.